- 翰林提供學(xué)術(shù)活動(dòng)、國際課程、科研項(xiàng)目一站式留學(xué)背景提升服務(wù)!
- 400 888 0080
CIE A Level Chemistry復(fù)習(xí)筆記7.6.5 Relative Basicity of Ammonia, Ethylamine & Phenylamine
Relative Basicity of Aqueous Ammonia, Ethylamine & Phenylamine
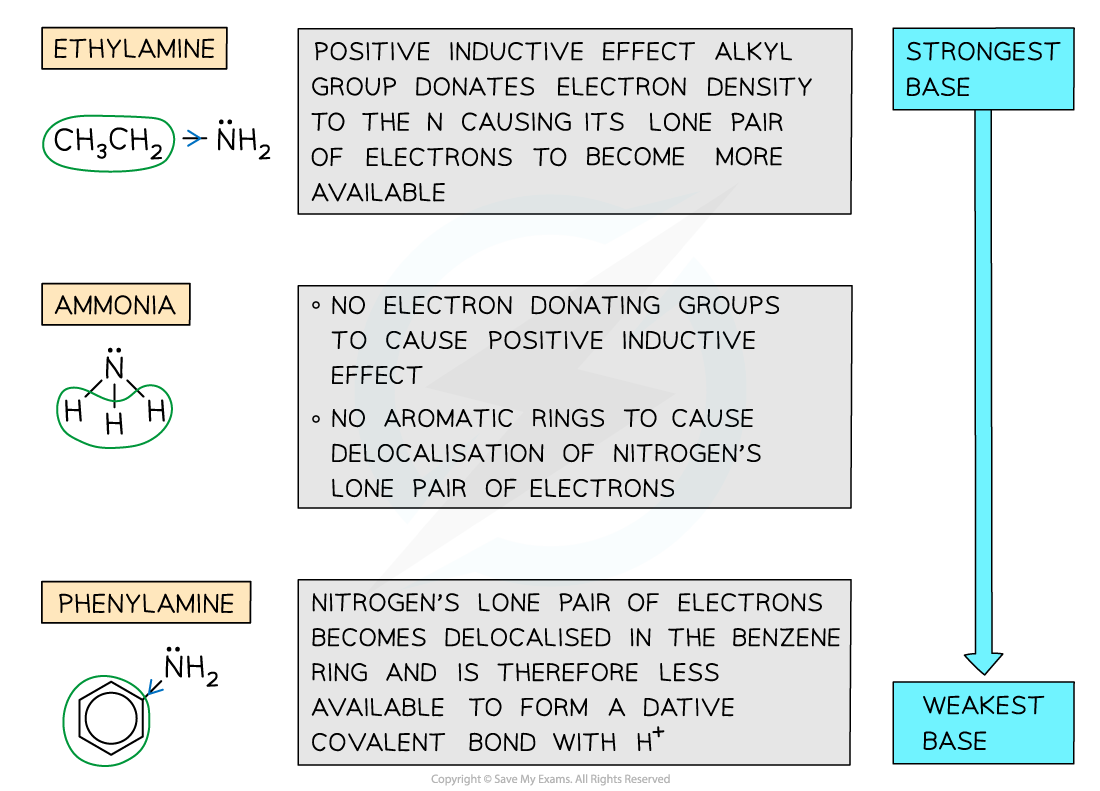
- Ammonia and amines act as?bases?as they can donate their lone pair of electrons to form a dative covalent bond with a proton
- The?basicity?of the amines depends on how readily available their lone pair of electrons is
- Electron-donating?groups (such as alkyl groups) increase the electron density on the nitrogen atom and cause the lone pair of electrons to become?more?available for dative covalent bonding
- The amine becomes?more?basic
- Delocalisation?of the lone pair of electrons into an aromatic ring (such as a benzene ring) causes the lone pair of electrons to become?less?available for dative covalent bonding
- The amine becomes?less?basic
Comparing basicity of ammonia, ethylamine & phenylamine
- The order of basicity of ammonia, ethylamine and phenylamine is as follows:
Ethylamine > ammonia > phenylamine
STRONGEST BASE???????????????????????????????????????????????????? ? WEAKEST BASE
- This trend can be explained by looking at the groups attached to the amine (-NH2) group
- In ethylamine, the electron-donating alkyl group donates electron density to the nitrogen atom causing its lone pair to become more available to form a dative covalent bond with a proton
- Ammonia lacks an electron-donating group hence it is less basic than ethylamine however it is more basic than phenylamine as the lone pair on the nitrogen is?not delocalised
- In phenylamine the lone pair of electrons overlap with the conjugated system on the benzene ring and become delocalised; As a result, the lone pair of electrons become less readily available to form a bond with a proton
Trends in the basicity of ammonia, ethylamine, and phenylamine
轉(zhuǎn)載自savemyexams

最新發(fā)布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









