- 翰林提供學(xué)術(shù)活動(dòng)、國(guó)際課程、科研項(xiàng)目一站式留學(xué)背景提升服務(wù)!
- 400 888 0080
IB DP Chemistry: HL復(fù)習(xí)筆記11.2.1 Recording Data
Qualitative & Quantitative data
- When recording results of experiment, both?quantitative?and?qualitative?data should be obtained
- Quantitative data?is obtained from measurements whereas?qualitative data?is non-numerical information that comes from observations
- Quantitative data?is always associated with random errors/uncertainties, determined by the apparatus, and by human limitations such as reaction time
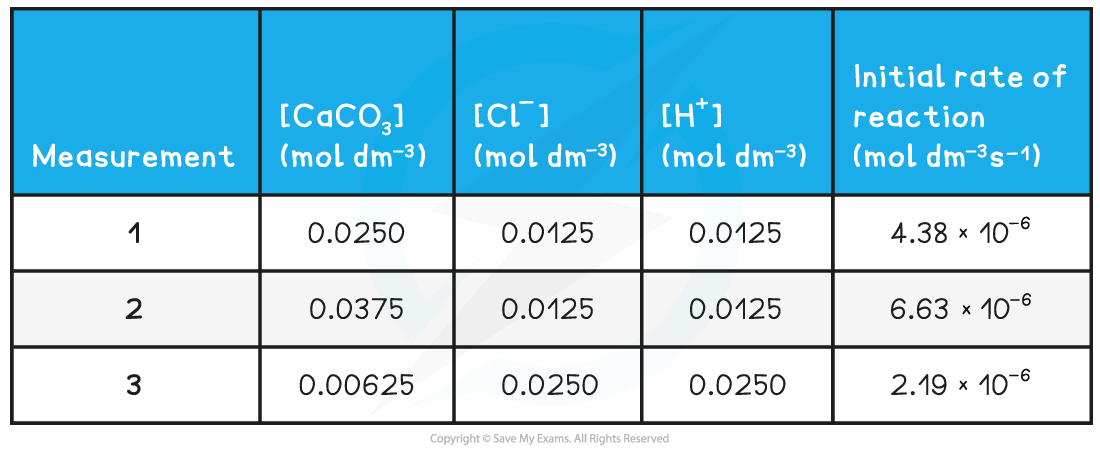
- Where there are several items of data you should record your data in a table with appropriate?headings?and?units:
Data Table showing headings and units
Uncertainties
- Uncertainties?are the same as?random errors
- Uncertainties?express the confidence to which the measurement can be taken
- Treatment of?uncertainties?depends on the type of instrument used
Using analogue instruments
- Any instruments that have an?analogue scale, the uncertainty is taken as?half the smallest division?on the scale
- For example,
- A thermometer that reads to 1oC, the uncertainty would be?+0.5?o?C
- A burette that reads to 0.10 mL, the uncertainty would be?+0.05 mL
Using digital instruments
- Any instruments that have a?digital?scale, the uncertainty is taken as the?smallest division?on the scale
- For example,
- An electronic balance that reads to 0.01 g, the uncertainty would be?+0.01 g
Other uncertainties
- Other sources of uncertainty can arise where the judgement of the experimenter is needed to determine a changing property
- For example,
- Judging the end point of a?titration?by looking at the colour of the?indicator
- Controlling a stopwatch in a rate of reaction experiment
- Deciding when to extinguish the flame in an?enthalpy of combustion?experiment
- These uncertainties are very difficult to quantify, but they should be commented on as a source of error in an?evaluation
Exam Tip
Notice that when recording the measurement you should always record it to the same level of precision as the uncertainty. The measurement cannot be any more or less precise than the uncertainty. Even though a burette reads to 0.1 mL, it must be recorded as 0.10 mL, so the last digit is always a 0 or a 5
轉(zhuǎn)載自savemyexams

最新發(fā)布
? 2025. All Rights Reserved. 滬ICP備2023009024號(hào)-1









