- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
AQA A Level Chemistry復習筆記5.4.2 Standard Electrode Potentials
Standard Electrode Potentials
Standard electrode potential
- The position of equilibrium and therefore the electrode potential depends on factors such as:
- Temperature
- Pressure of gases
- Concentration of reagents
- So, to be able to compare the electrode potentials of different species, they all have to be measured against a common reference or standard
- Standard?conditions?also have to be used when comparing electrode potentials
- These standard conditions are:
- Ion concentration of 1.00 mol dm-3
- A temperature of 298 K
- A pressure of 100 kPa
- Standard measurements are made using a?high resistance voltmeter?so that no current flows and the maximum potential difference is achieved
- The electrode potentials are measured relative to a?standard hydrogen electrode
- The standard hydrogen electrode is given a value of 0.00 V, and all other electrode potentials are compared to this standard
- This means that the electrode potentials are always referred to as a?standard electrode potential?(E?)
- The?standard electrode potential?(E?)?is the potential difference ( sometimes called voltage) produced when a?standard half-cell?is connected to a?standard hydrogen cell?under standard conditions
- For example, the standard electrode potential of bromine suggests that relative to the hydrogen half-cell it is more likely to get reduced, as it has a?more positive?E??value
Br2(l) + 2e–?? 2Br–(aq)? ? ? ??E??= +1.09 V
2H+(aq) + 2e–?? H2(g)? ? ? ??E??= 0.00 V
- The standard electrode potential of sodium, on the other hand, suggests that relative to the hydrogen half-cell it is less likely to get reduced as it has a?more negative?E??value
Na+?(aq) + e–?? Na(s)? ? ? ??E??= -2.71 V
2H+?(aq) + 2e–?? H2(g)? ? ? ??E??= 0.00 V
Standard Hydrogen Electrode
- The?standard hydrogen electrode?is a half-cell used as a?reference electrode?and consists of:
- Hydrogen gas in equilibrium with H+?ions of concentration 1.00 mol dm-3?(at 100 kPa)
2H+?(aq) + 2e-?? H2?(g)
-
- An?inert?platinum?electrode that is in contact with the hydrogen gas and H+ ions
- When the standard hydrogen electrode is connected to another half-cell, the?standard electrode potential?of that half-cell can be read off a high resistance voltmeter
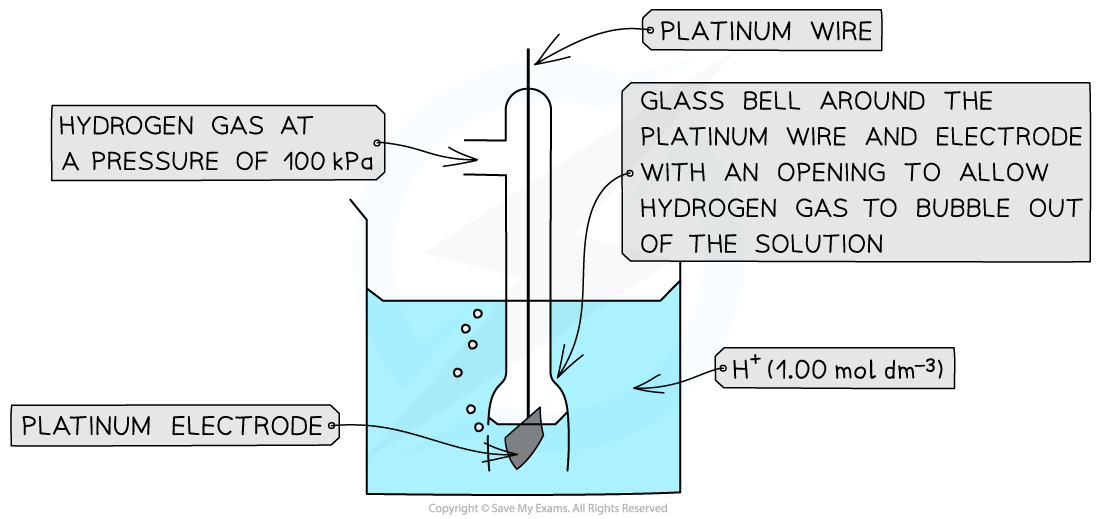
The standard electrode potential of a half-cell can be determined by connecting it to a standard hydrogen electrode
- There are three different types of half-cells that can be connected to a standard hydrogen electrode
- A metal / metal ion half-cell
- A non-metal / non-metal ion half-cell
- An ion / ion half-cell (the ions are in different oxidation states)
Metal / metal-ion half-cell
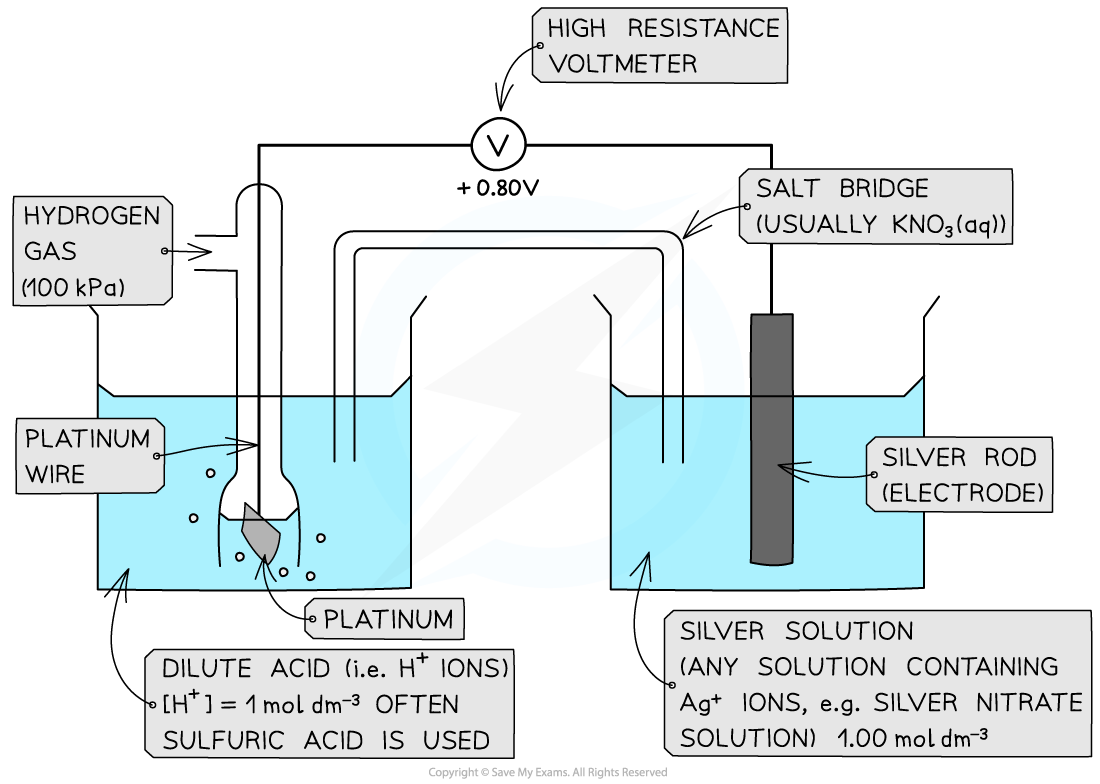
Example of a metal / metal ion half-cell connected to a standard hydrogen electrode
- An example of a metal/metal ion half-cell is the Ag+/ Ag half-cell
- Ag is the metal
- Ag+?is the metal ion
- This half-cell is connected to a?standard hydrogen electrode?and the two half-equations are:
Ag+?(aq) + e-?? Ag (s)? ? ? ??E??= + 0.80 V
2H+?(aq) + 2e-?? H2?(g)? ? ? ??E??= 0.00 V
- Since the Ag+/ Ag half-cell has a more positive?E??value, this is the?positive pole?and the H+/H2?half-cell is the?negative?pole
- The?standard cell potential (Ecell?) is?Ecell??= (+ 0.80) - (0.00)?= + 0.80 V
- The Ag+?ions are more likely to get?reduced?than the H+?ions as it has a greater?E??value
- Reduction occurs at the?positive electrode
- Oxidation occurs at the?negative electrode
Non-metal / non-metal ion half-cell
- In a?non-metal / non-metal ion?half-cell,?platinum?wire or foil is used as an electrode to make electrical contact with the solution
- Like graphite, platinum is inert and does not take part in the reaction
- The redox equilibrium is established on the platinum surface
- An example of a non-metal / non-metal ion is the Br2?/ Br-?half-cell
- Br2?is the non-metal
- Br-?is the non-metal ion
- The half-cell is connected to a?standard hydrogen electrode?and the two half-equations are:
Br2?(aq) + 2e-?? 2Br-?(aq)? ? ? ??E??= +1.09 V
2H+?(aq) + 2e-?? H2?(g)? ? ? ??E??= 0.00 V
- The Br2?/ Br-?half-cell is the?positive pole?and the H+?/ H2?is the?negative?pole
- The?Ecell??is:?Ecell??= (+ 1.09) - (0.00)?= + 1.09 V
- The Br2?molecules are more likely to get?reduced?than H+?as they have a greater?E??value
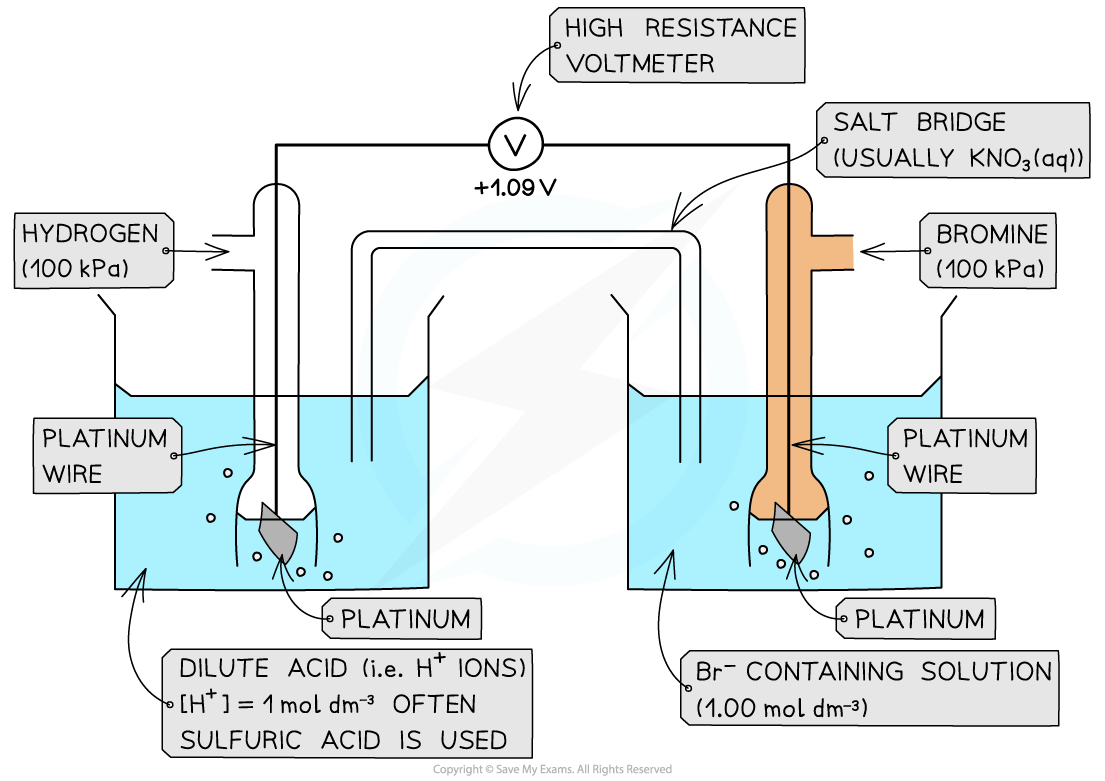
Example of a non-metal / non-metal ion half-cell connected to a standard hydrogen electrode
Ion / Ion half-cell
- A?platinum electrode?is again used to form a half-cell of ions that are in?different oxidation states
- An example of such a half-cell is the MnO4-?/ Mn2+?half-cell
- MnO4-?is an ion containing Mn with oxidation state +7
- The Mn2+?ion contains Mn with oxidation state +2
- This half-cell is connected to a?standard hydrogen electrode?and the two half-equations are:
MnO4-?(aq) + 8H+?(aq) + 5e-?? Mn2+?(aq) + 4H2O (l)? ? ? ?E??= +1.52 V
2H+?(aq) + 2e-?? H2?(g)? ? ? ?E??= 0.00 V
- The H+?ions are also present in the half-cell as they are required to convert MnO4-?into Mn2+?ions
- The MnO4-?/ Mn2+?half-cell is the?positive pole?and the H+?/ H2?is the?negative?pole
- The?Ecell??is?Ecell??= (+ 1.09) - (0.00)?= + 1.09 V
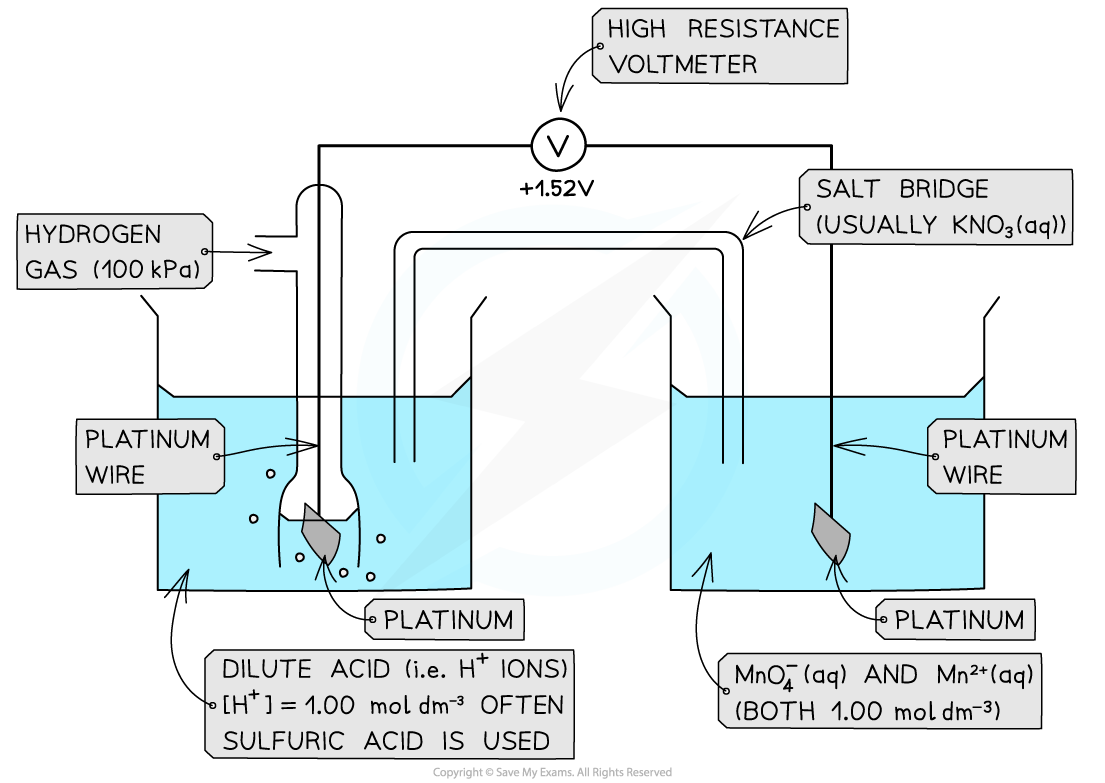
Ions in solution half cell
Calculating EMF
Standard cell potential
- Once the?E??of a half-cell is known, the?potential difference?or?voltage?or?emf?of an?electrochemical cell?made up of any two half-cells can be calculated
- These could be?any?half-cells and neither have to be a standard hydrogen electrode
- The?standard cell potential?(Ecell?) can be calculated by?subtracting?the?less?positive?E??from the?more positive?E??value
- The half-cell with the more positive?E??value will be the?positive?pole
- By convention this is shown on the right hand side in a conventional cell diagram, so is termed?Eright?
- The half-cell with the less positive?E??value will be the?negative?pole
- By convention this is shown on the left hand side in a conventional cell diagram, so is termed?Eleft?
- The half-cell with the more positive?E??value will be the?positive?pole
Ecell??=?Eright??-?Eleft?
-
- Since oxidation is always on the left and reduction on the right, you can also use this version
Ecell??=?Ereduction??-?Eoxidation
Worked Example
Calculating the standard cell potential
Calculate the standard cell potential for the electrochemical cell below and explain why the Cu2+?/ Cu half-cell is the positive pole. The half-equations are as follows:
Cu2+(aq) + 2e-?? Cu(s)? ? ??E??= +0.34 V
Zn2+(aq) + 2e-?? Zn(s)? ? ??E??= ?0.76 V
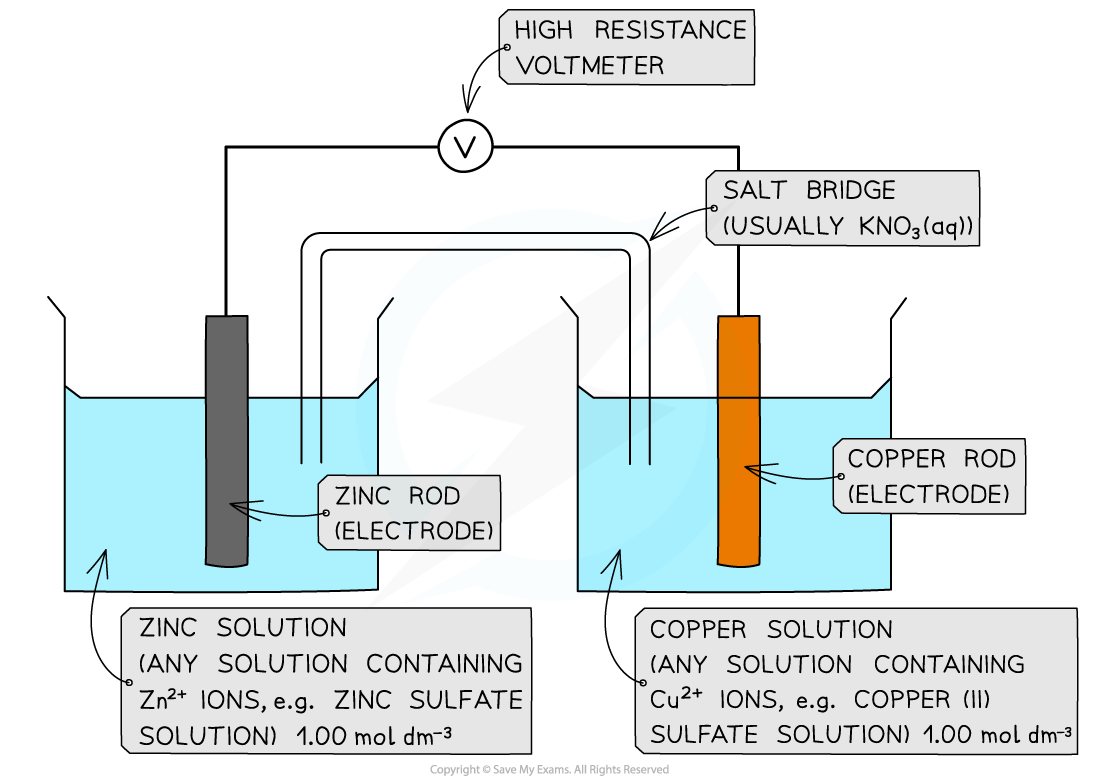
Answer
Step 1:?Calculate the standard cell potential. The copper is more positive so must be the right hand side.
Ecell??=?Eright??-?Eleft?
Ecell??= (+0.34) - (-0.76)
= +1.10 V
The voltmeter will therefore give a value of +1.10 V
Step 2:?Determine the positive and negative poles
The Cu2+?/ Cu? half-cell is the?positive?pole as its?E??is more positive than the?E??value of the Zn2+?/ Zn half-cell
Exam Tip
A helpful mnemonic for remembering redox in cells
Lio the lion goes Roor!
Lio stands for 'Left Is Oxidation' and he is saying ROOR because that is the order of species in the cell:
Reduced/Oxidised (salt bridge) Oxidised/Reduced
轉載自savemyexams

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









