- 翰林提供學(xué)術(shù)活動(dòng)、國(guó)際課程、科研項(xiàng)目一站式留學(xué)背景提升服務(wù)!
- 400 888 0080
CIE A Level Chemistry復(fù)習(xí)筆記7.5.4 Relative Acidities of Chlorine-substituted Carboxylic Acids
Relative Acidities of Chlorine-Substituted Carboxylic Acids
- Electron-withdrawing?groups bonded to the carbon attached to the -COOH group make the carboxylic acids?stronger acids
- This is because the O-H bond in the?undissociated acid molecule?is even further weakened as the electron-withdrawing group draws even more electron density away from this bond
- Furthermore, the electron-withdrawing groups extend the?delocalisation?of the negative charge on the -COO-?group of the carboxylate ion
- The -COO-?group is now even more stabilised and is less likely to bond with an H+?ion
- Chlorine-substituted carboxylic acids?are examples of carboxylic acids with electron-withdrawing groups
pKa?values of ethanoic acid and chlorine-substituted derivatives table
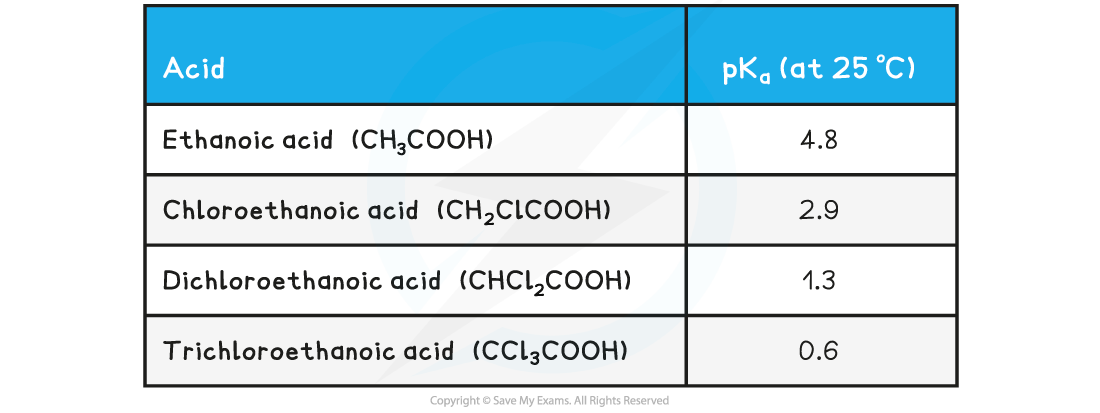
- The pKa?values of ethanoic acid and?chloro-substituted derivatives?show that the?more?electron-withdrawing groups there are on the carbon attached to the -COOH group, the?stronger?the acid
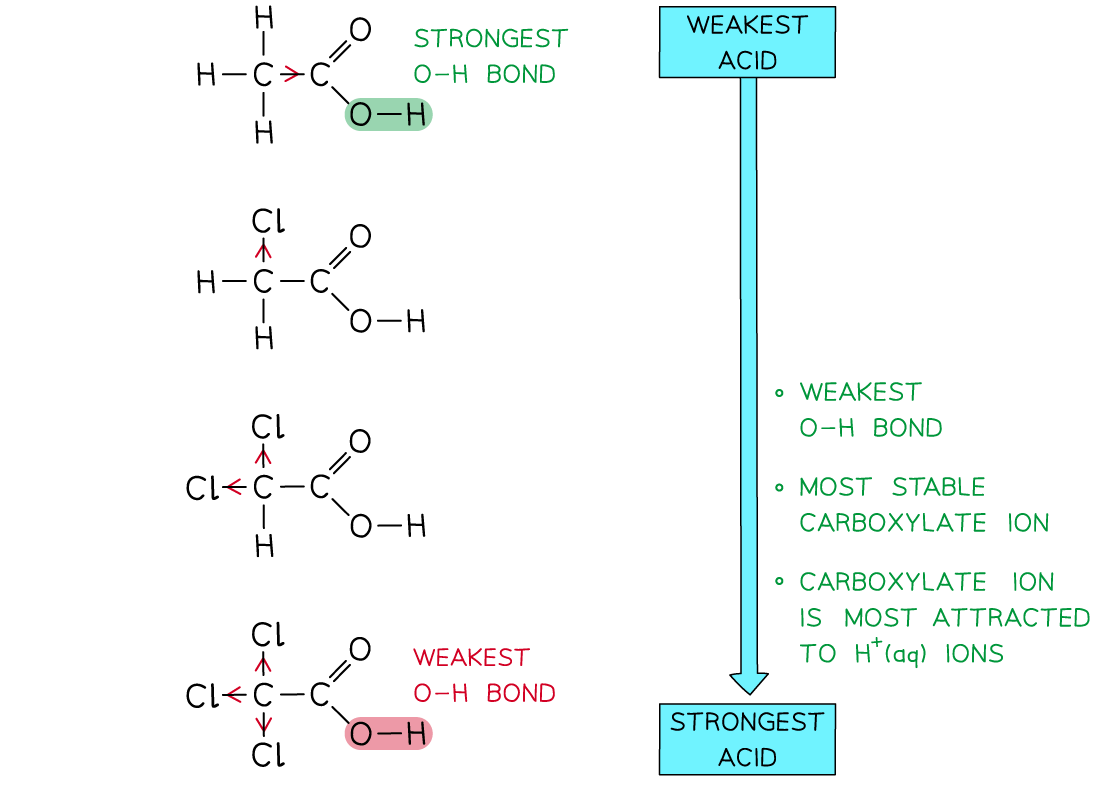
The more chlorine atoms there are in the carboxylic acids, the stronger the acid is
- Trichloroethanoic acid is the?strongest acid?as:
- The O-H bond in CCl3COOH is the?weakest?since there are?three?very strong electronegative Cl atoms withdrawing electron density from the -COOH group
- When the O-H is broken to form the carboxylate (-COO-) ion, the charge density is further spread out by the three electron-withdrawing Cl atoms
- The carboxylate ion is so?stabilised?that it is less attracted to H+?ions
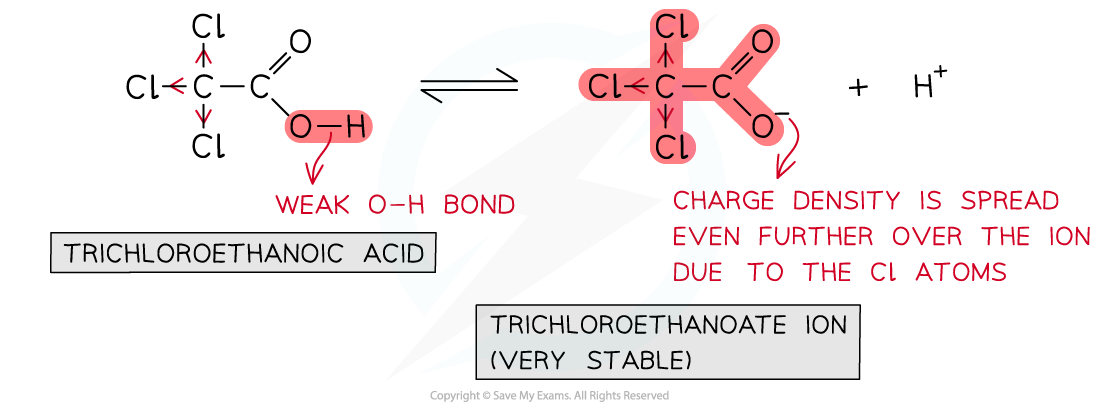
Relative acidity of trichloroethanoic acid
- Ethanoic acid is the?weakest acid?as:
- It contains an?electron-donating?methyl group which?strengthens?the O-H bond
- The methyl group?donates?negative charge towards the -COO-?group which becomes more likely to accept an H+?ion
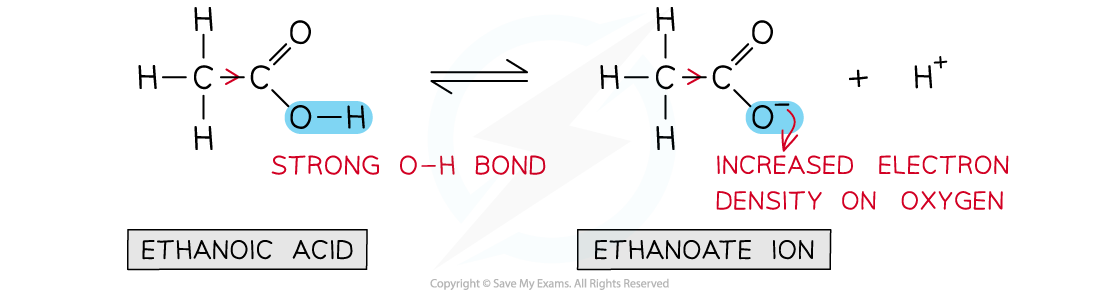 Relative acidity of ethanoic acid
Relative acidity of ethanoic acid
轉(zhuǎn)載自savemyexams

最新發(fā)布
? 2025. All Rights Reserved. 滬ICP備2023009024號(hào)-1









