- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
CIE A Level Chemistry復習筆記7.2.1 Reactions of Arenes
Reactions of Arenes
- Arenes are very stable compounds due to the?delocalisation of π electrons?in the ring
- This is because the negative charge is spread out over the molecule instead of being confined to a small area
- During chemical reactions such as?substitution reactions, this delocalised ring is maintained
- Addition reactions?however,?disrupt?the?aromatic stabilisation
- Arenes undergo a series of reactions including:
- Substitution
- Nitration
- Friedel-Crafts alkylation
- Friedel-Crafts acylation
- Complete Oxidation
- Hydrogenation
Substitution
- Halogenation?reactions are examples of?electrophilic substitution?reactions
- Arenes undergo?substitution?reactions with chlorine (Cl2) and bromine (Br2) in the presence of anhydrous AlCl3?or AlBr3?catalyst?respectively to form?halogenoarenes?(aryl halides)
- The chlorine or bromine act as an?electrophile?and replaces a hydrogen atom on the benzene ring
- The catalyst is required for the reaction to take place, due to the stability of the benzene structure

Arenes undergo substitution reactions with halogens to form aryl halides
- Alkylarenes?such as methylbenzene undergo halogenation on the 2 or 4 positions
- This is due to the?electron-donating?alkyl groups which activate these positions
- Phenol (C6H5OH) and phenylamine (C6H5NH2) are also activated in the 2 and 4 positions
- The halogenation of alkylarenes therefore result in the formation of?two products
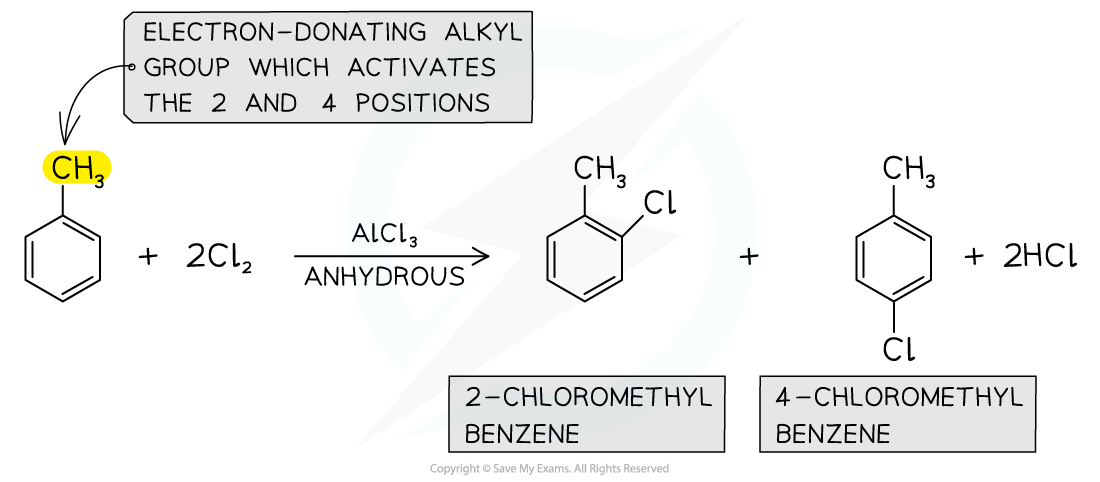
Alkylarenes are substituted on the 2 or 4 position
- Multiple substitutions?occur when?excess?halogen is used
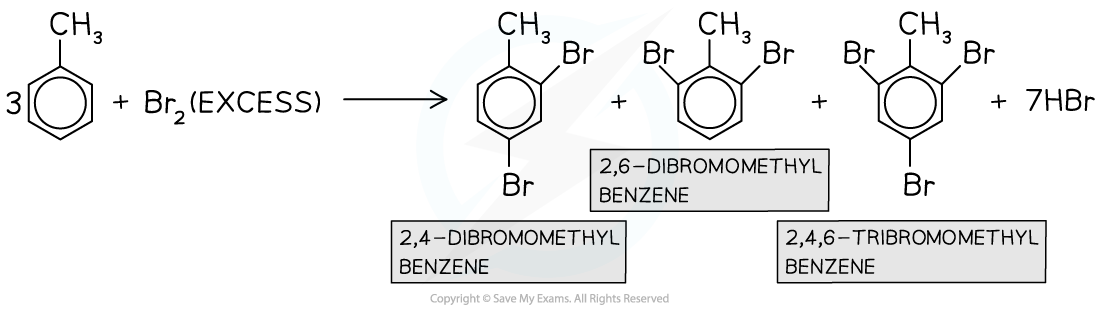
In the presence of excess halogen, multiple substitutions occur
Nitration
- Another example of a substitution reaction is the?nitration?of arenes
- In these reactions, a nitro (-NO2) group replaces a hydrogen atom on the arene
- The benzene is reacted with a mixture of concentrated nitric acid (HNO3) and concentrated sulfuric acid (H2SO4) at a temperature between 25 and 60?oC

Nitration of benzene
- Again, due to the?electron-donating alkyl groups in alkylarenes, nitration of methylbenzene will occur on the 2 and 4 position
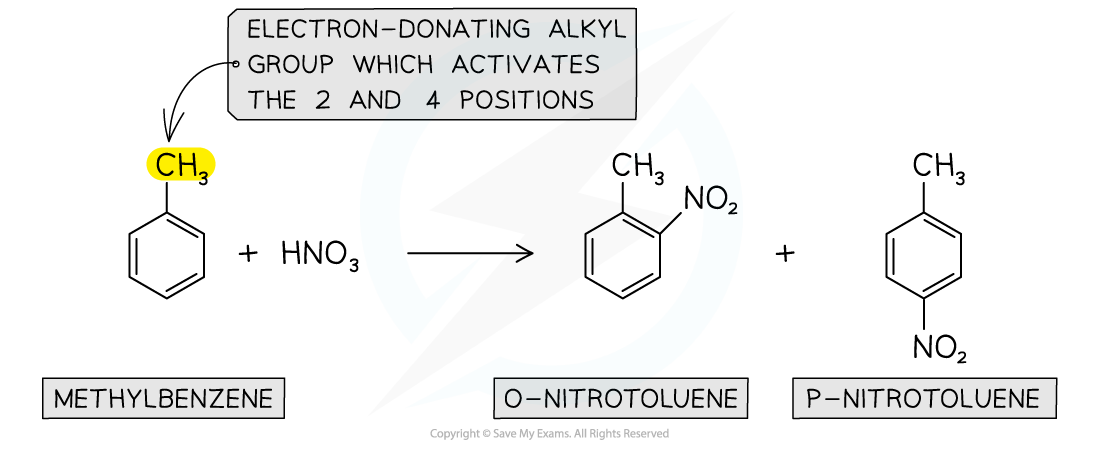
Nitration of alkylarenes
Friedel-Crafts reactions
- Friedel-Crafts reactions are also?electrophilic substitution?reactions
- Due to the aromatic stabilisation in arenes, they are often?unreactive
- To use arenes as?starting materials?for the synthesis of other organic compounds, their structure, therefore, needs to be changed to turn them into more reactive compounds
- Friedel-Crafts reactions can be used to substitute a hydrogen atom in the benzene ring for an?alkyl group?(Friedel-Crafts alkylation) or an?acyl group?(Friedel-Crafts acylation)
- Like any other electrophilic substitution reaction, the Friedel-Crafts reactions consist of three steps:
- Generating the electrophile
- Electrophilic attack on the benzene ring
- Regenerating aromaticity of the benzene ring
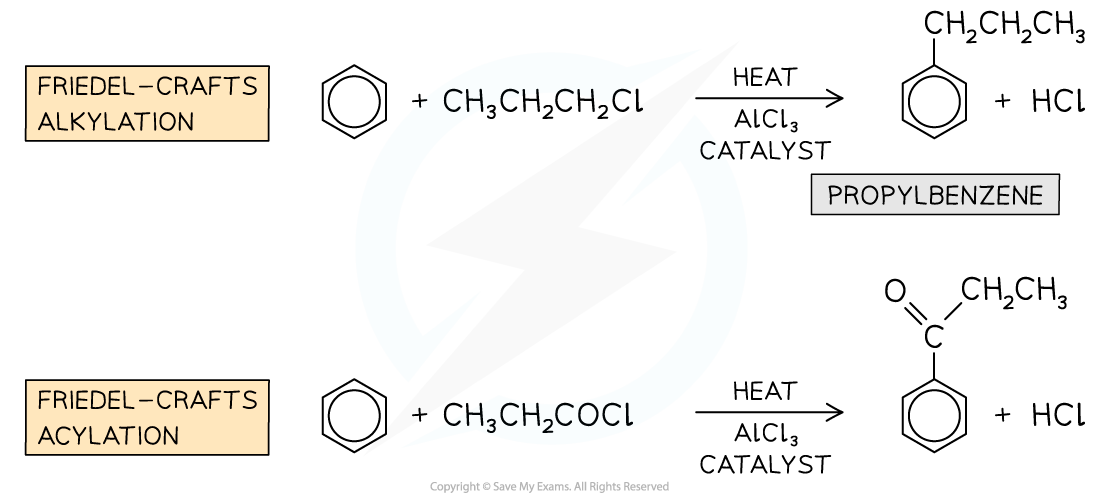
Examples of Friedel-Crafts alkylation and acylation reactions
Friedel-Crafts alkylation
- In this type of Friedel-Crafts reaction, an?alkyl chain?is substituted into the benzene ring
- The benzene ring is reacted with a chloroalkane in the presence of an AlCl3?catalyst
- An example of an alkylation reaction is the reaction of benzene with chloropropane (CH3CH2CH2Cl) to form propylbenzene
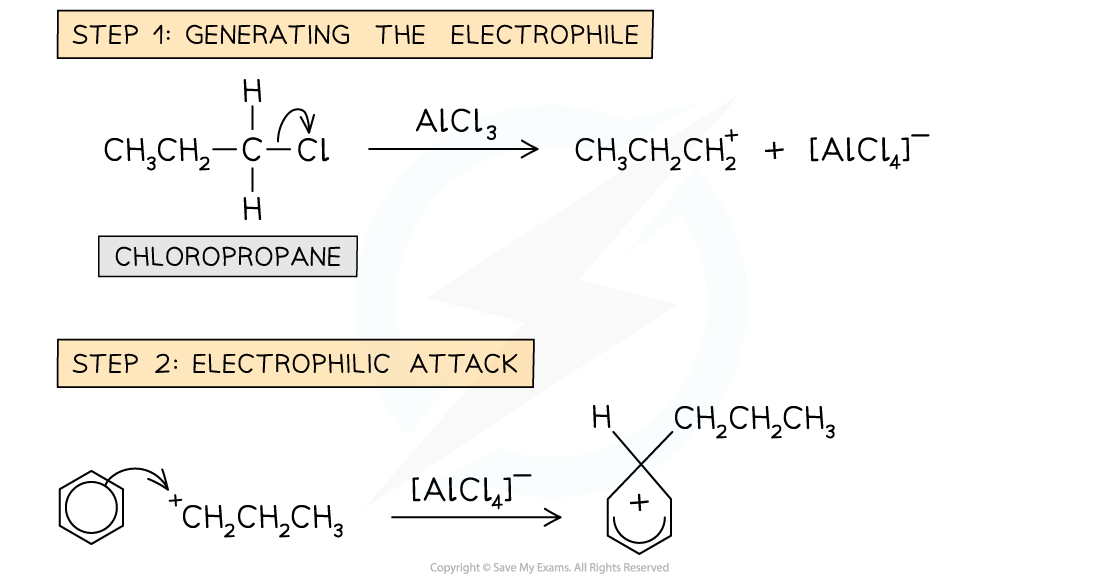
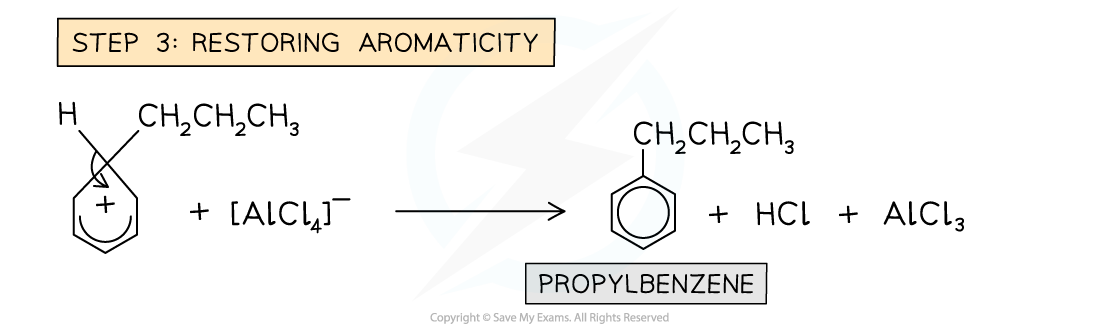
Example of a Friedel-Crafts alkylation reaction
Friedel-Crafts acylation
- In the Friedel-Crafts acylation reaction, an?acyl group?is substituted into the benzene ring
- An acyl group is an alkyl group containing a carbonyl, C=O group
- The benzene ring is reacted with an acyl chloride in the presence of an AlCl3?catalyst
- An example of an acylation reaction is the reaction of methylbenzene with propanoyl chloride to form an acyl benzene
- Note that the acyl group is on the 4 position due to the -CH3?group on the benzene
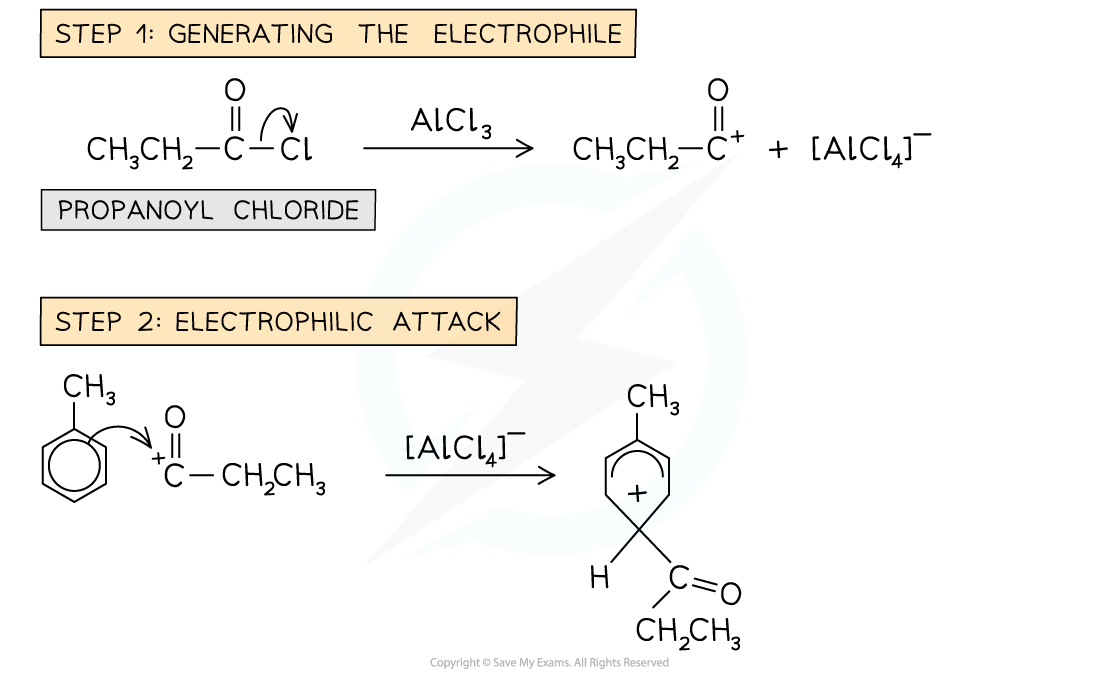
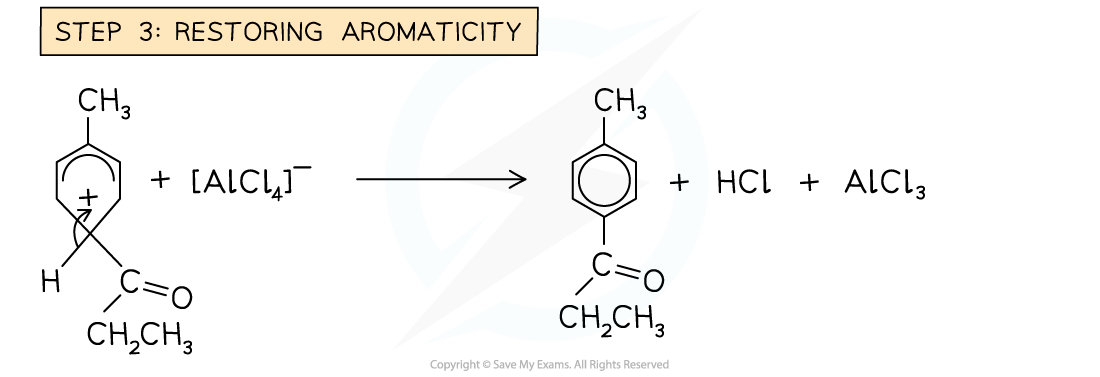
Example of a Friedel-Crafts acylation reaction
Complete oxidation
- Normally, alkanes are not?oxidised?by?oxidising agents?such as potassium manganate(VII) (KMnO4)
- However, the presence of the benzene ring in?alkyl arenes?affect the properties of the alkyl side-chain
- The alkyl side-chains in alkyl arenes are?oxidised?to?carboxylic acids?when?refluxed?with?alkaline potassium manganate(VII)?and then?acidified?with?dilute sulfuric acid?(H2SO4)
- For example, the complete oxidation of?ethylbenzene?forms?benzoic acid

The complete oxidation of alkyl side-chains in arenes gives a carboxylic acid
Hydrogenation
- The hydrogenation of benzene is an?addition reaction
- Benzene is?heated?with?hydrogen gas?and a?nickel?or?platinum catalyst?to form?cyclohexane

Hydrogenation of benzene
- The same reaction occurs when?ethylbenzene?undergoes hydrogenation to form?cycloethylbenzene

Hydrogenation of methylbenzene
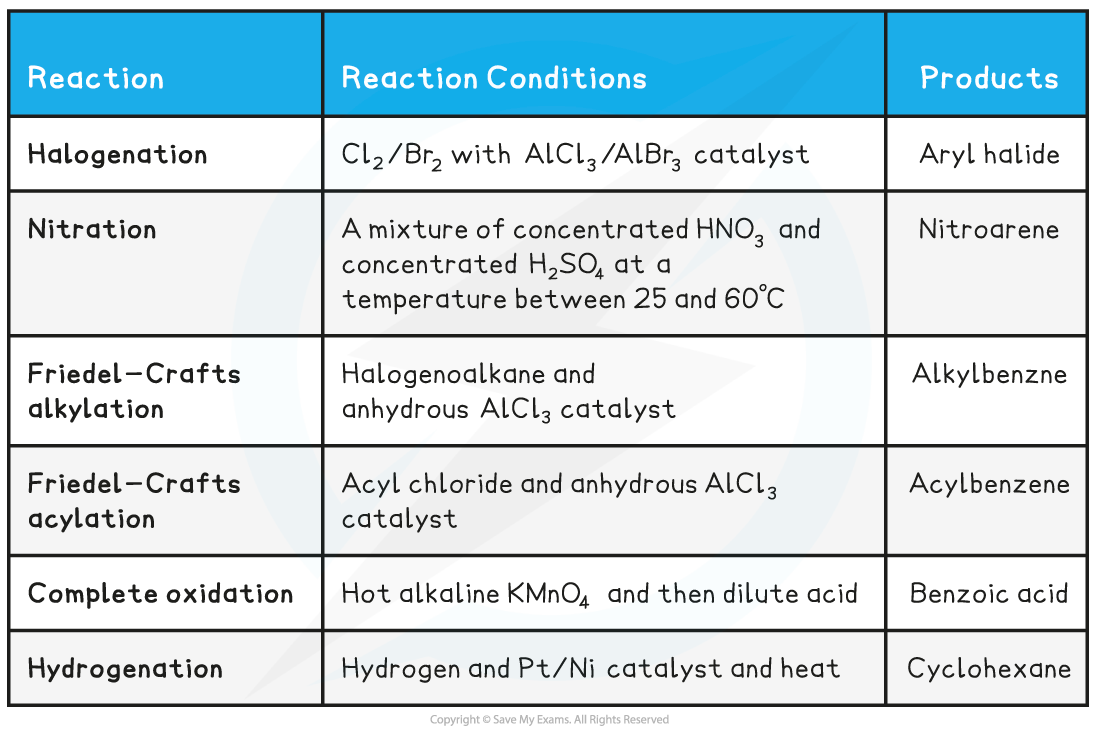
Summary of Reactions of Arenes Table
轉載自savemyexams

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









