- 翰林提供學(xué)術(shù)活動(dòng)、國(guó)際課程、科研項(xiàng)目一站式留學(xué)背景提升服務(wù)!
- 400 888 0080
CIE A Level Chemistry復(fù)習(xí)筆記1.1.9 Determining Electronic Configurations
Determining Electronic Configurations
- Writing out the?electronic?configuration?tells us how the electrons in an atom or ion are arranged in their shells, subshells and orbitals
- This can be done using the?full?electron configuration or the?shorthand?version
- The?full?electron configuration describes the arrangement of all electrons from the 1s subshell up
- The?shorthand?electron configuration includes using the symbol of the nearest preceding?noble?gas?to account for however many electrons are in that noble gas
- Ions?are formed when atoms?lose?or?gain?electrons
- Negative ions are formed by?adding?electrons to the outer subshell
- Positive ions are formed by?removing?electrons from the outer subshell
- The transition metals?fill?the 4s subshell before the 3d subshell but?lose?electrons from the 4s first and not from the 3d subshell (the 4s subshell is lower in energy)
- The Periodic Table is split up into four main blocks depending on their electronic configuration:
- s block elements
- Have their valence electron(s) in an s orbital
- p block elements
- Have their valence electron(s) in a p orbital
- d block elements
- Have their valence electron(s) in a d orbital
- f block elements
- Have their valence electron(s) in an f orbital
- s block elements
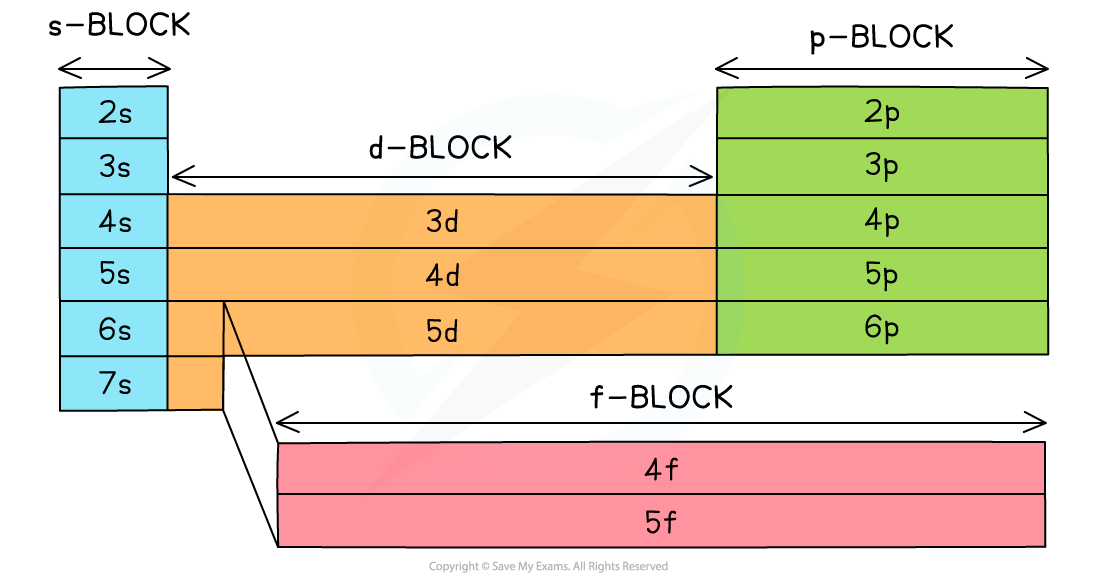
The elements can be divided into four blocks according to their outer shell electron configuration
Exceptions
- Chromium and copper have the following electron configurations, which are different to what you may expect:
- Cr is [Ar] 3d5?4s1?not?[Ar] 3d4?4s2
- Cu is [Ar] 3d10?4s1?not?[Ar] 3d9?4s2
- This is because the [Ar] 3d5?4s1?and [Ar] 3d10?4s1?configurations are?energetically stable
Worked example: Electron configuration
Answer
Answer 1:?Potassium has 19 electrons so the?full electronic configuration?is:
1s2?2s2?2p6?3s2?3p6?4s1
The 4s orbital is lower in energy than the 3d subshell and is therefore filled first
The nearest preceding noble gas to potassium is?argon?which accounts for 18 electrons so the?shorthand?electron configuration?is:
[Ar] 4s1
Answer 2:?Calcium has 20 electrons so the?full?electronic?configuration?is:
1s2?2s2?2p6?3s23p6?4s2
The 4s orbital is lower in energy than the 3d subshell and is therefore filled first
The?shorthand?version is [Ar] 4s2?since argon is the nearest preceding noble gas to calcium which accounts for 18 electrons
Answer 3:?Gallium has 31 electrons so the?full?electronic?configuration?is:
[Ar] 3d10?4s2?4p1
Answer 4:?What this means is that if you ionise calcium and remove two of its outer electrons, the electronic configuration of the Ca2+?ion is identical to that of argon
Ca2+?is 1s22s2?2p6?3s2?3p6
Ar?is also 1s2?2s2?2p6?3s23p6
轉(zhuǎn)載自savemyexams

最新發(fā)布
? 2025. All Rights Reserved. 滬ICP備2023009024號(hào)-1









