- 翰林提供學(xué)術(shù)活動、國際課程、科研項目一站式留學(xué)背景提升服務(wù)!
- 400 888 0080
CIE A Level Chemistry復(fù)習(xí)筆記1.1.1 Particles in the Atom & Atomic Structure
Structure of an Atom
- All matter is composed of?atoms, which are the smallest parts of an element that can take place in?chemical reactions
- Atoms are mostly made up of?empty?space?around a very small, dense?nucleus?that contains?protons?and?neutrons
- The nucleus has an overall?positive?charge
- The protons have a positive charge and the neutrons have a neutral charge
- Negatively?charged?electrons are found in orbitals in the empty space around the nucleus
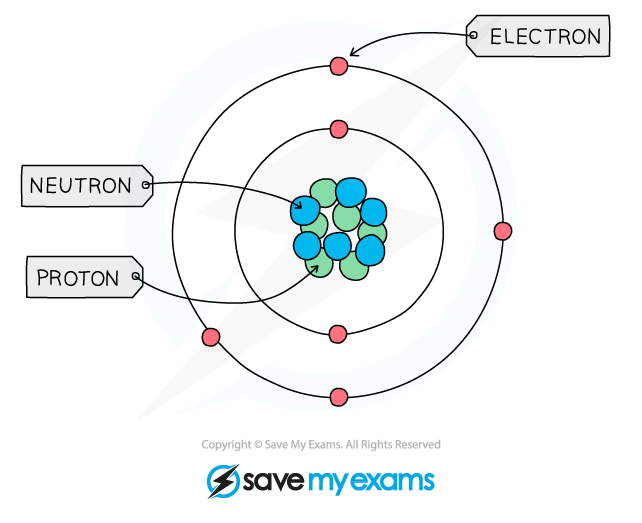
The basic structure of an atom (not to scale)
Subatomic Particles
- The?protons,?neutrons?and?electrons?that an atom is made up of are called?subatomic particles
- These subatomic particles are so small that it is not possible to measure their masses and charges using?conventional?units?(such as grams or coulombs)
- Instead, their masses and charges are compared to each other, and so are called?‘relative?atomic?masses’?and ‘relative atomic charges’
- These are not actual charges and masses but charges and masses of particles relative to each other
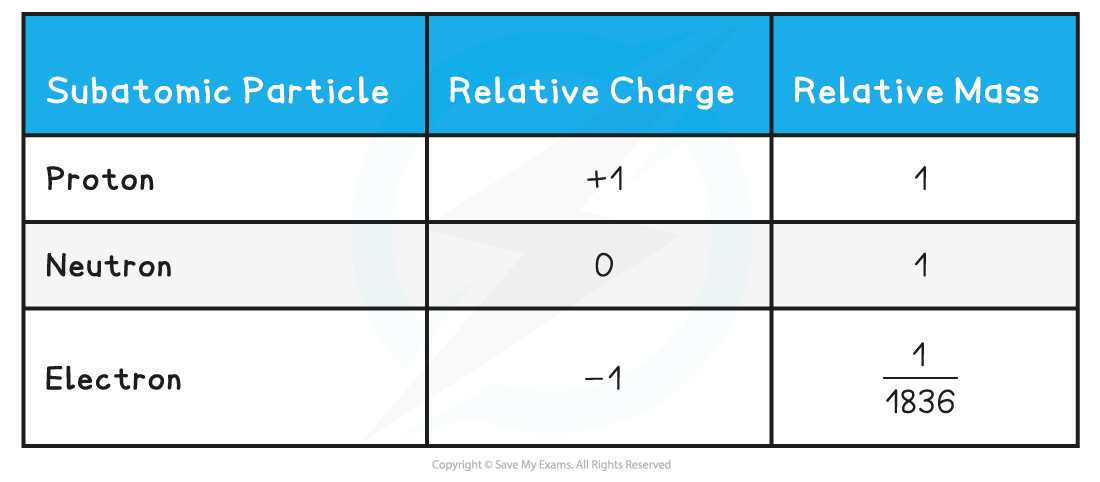
- Protons and neutrons have a very similar mass, so each is assigned a relative mass of?1
- Electrons are 1836 times smaller than a proton and neutron, and so their mass is often described as being negligible
- The relative mass and charge of the subatomic particles are:
Relative mass & charge of subatomic particles table
Exam Tip
You can see from the table how the?relative?mass?of an electron is?almost negligibleThe?charge?of a single?electron?is -1.602 x 10-19? coulombs, whereas the charge of a?proton?is +1.602 x 10-19? coulombs. So, relative to each other, their charges are -1 and +1 respectively
Atoms: Key Terms
- The?atomic number?(or?proton number) is the number of protons in the nucleus of an atom and has the?symbol?Z
- The atomic number is also equal to the number of electrons present in a?neutral?atom of an element
- E.g. the atomic number of lithium is 3, meaning that a neutral lithium atom has 3 protons and therefore, also has 3 electrons
- The?mass?number?(or?nucleon?number) is the total number of?protons?+?neutrons?in the nucleus of an atom, and has the??symbol?A
- The number of?neutrons?can be calculated by:
Number of neutrons = mass number - atomic number
-
- Protons and neutrons are also called?nucleons, because they are found in the nucleus
Exam Tip
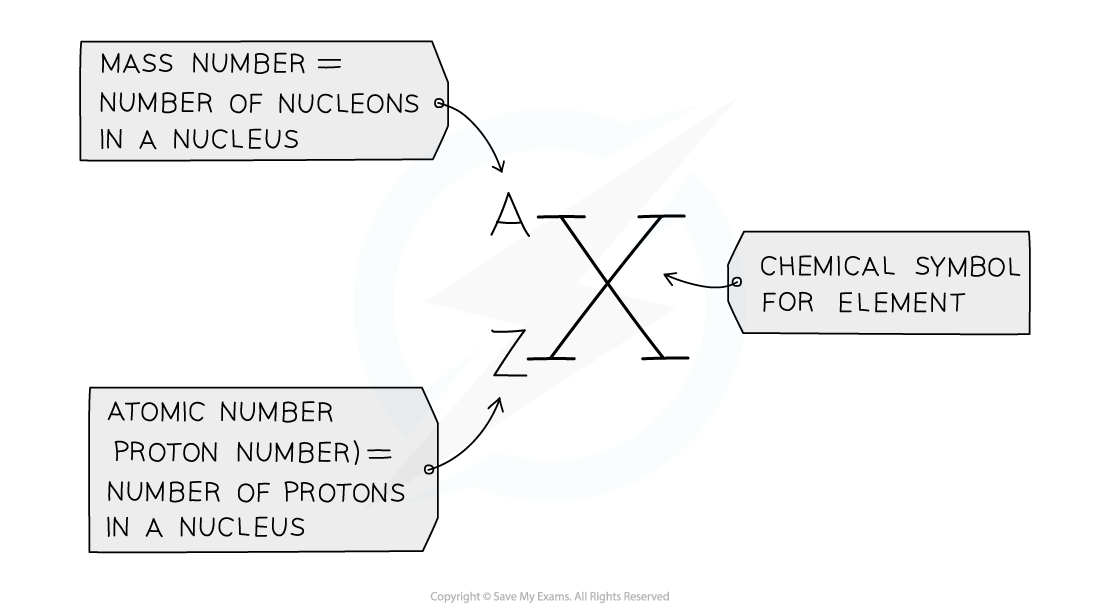
The mass (nucleon) and atomic (proton) number are given for each element in the Periodic Table
轉(zhuǎn)載自savemyexams

最新發(fā)布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









