- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
IB DP Chemistry: SL復習筆記5.3.4 Case Study: Ozone
Case study: Ozone & Bond Strength
- A study of?bond enthalpy?can explain why ozone and oxygen in the atmosphere play very different roles in the flow of energy
- These processes have a profound effect on the amount of solar radiation reaching ground level
- The structure of oxygen and ozone molecules influences the amount of energy needed to break their bonds:

The structure of oxygen and ozone
- The double bond in oxygen is stronger than the?delocalised π bonds?in ozone
- We say the?bond order?of oxygen is?2?and the?bond order?of ozone is?1.5
- Both bonds are broken by ultraviolet radiation but the bond in oxygen requires radiation of higher energy and shorter wavelength than the bond in ozone
- High energy UV radiation in the stratosphere breaks the oxygen-oxygen double bond creating oxygen atoms
O2?(g)??→?O??(g)?+ ?O??(g)????????????H +ve, UV light, λ < 242 nm
- These oxygen atoms have unpaired electrons- they are known as?free radicals
- The?free radicals?are highly reactive and quickly attack oxygen molecules forming ozone in an?exothermic?reaction, which raises the temperature of the stratosphere
OZONE FORMATION?? ? ? ? ? ? ? ? O??(g)?+ ?O2?(g)??→?O3?(g)??????????????H - ve
- Ozone requires less energy to break than oxygen
- It produces an oxygen molecule and an oxygen free radical:
OZONE DEPLETION? ? ? ? ? ? ? ? ?O3?(g)???→???O??(g)?+ ?O2?(g)? ???????????H +ve, UV light,?λ< 330 nm
- The radical reacts with another ozone molecule making two molecules of oxygen in an?exothermic?reaction
OZONE DEPLETION? ? ? ? ? ? ? ? ?O3?(g)??+?O??(g)?→???2O2?(g)? ???????????H - ve
- The temperature in the stratosphere is maintained by the balance of ozone formation and ozone depletion in a process known as the Chapman Cycle
- It is not a closed system as matter and energy flow in and out, but it is what is called a?steady state
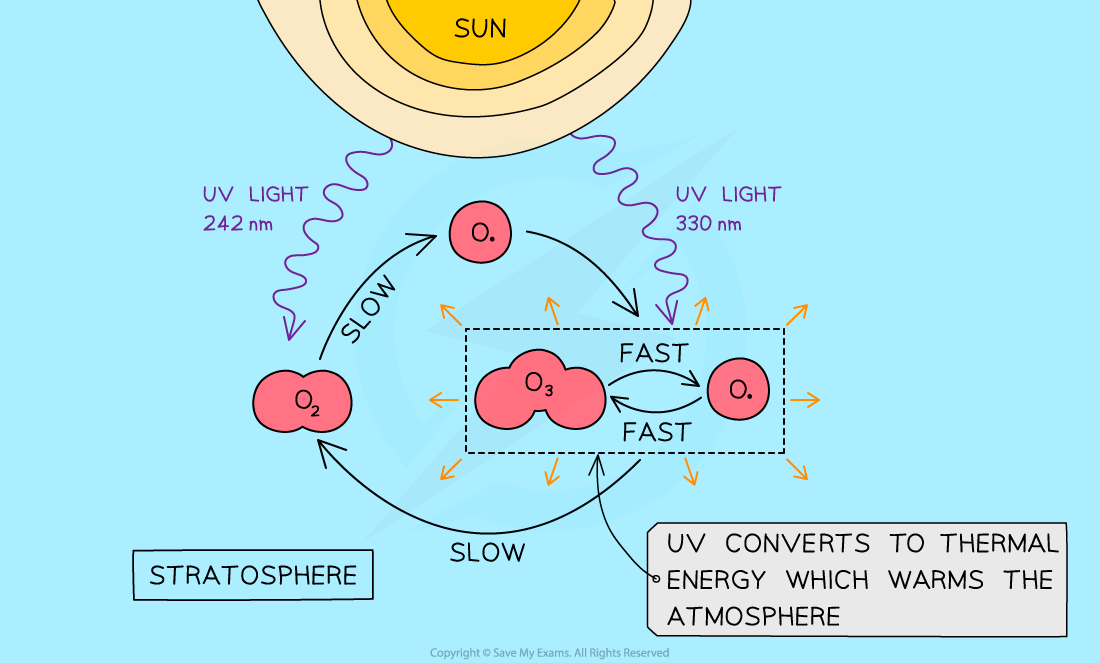
The Chapman cycle
- Unfortunately, chemicals we have introduced into the atmosphere have interfered with this steady state resulting in ozone depleting at a faster rate than it is replaced
- Amongst these chemicals are?chlorofluorocarbons (CFCs)?found in refrigerants, propellants and solvents
- CFCs?are greatly damaging to stratospheric ozone and have been largely replaced by safer alternatives following the 1985 Montreal Protocol
- The depletion of ozone has allowed greater amounts of harmful?UV light?to reach the surface of the Earth
- UV light?has been linked to greater incidence of skin cancer and cataracts as well as the destruction of phytoplankton and reduced plant growth
轉載自savemyexams

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









