- 翰林提供學術(shù)活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
IB DP Chemistry: HL復習筆記1.1.3 State Changes
State Changes
- Changes of state are?physical changes?that are reversible
- These changes do not change the chemical properties or chemical makeup of the substances involved
- Vaporization?includes?evaporation?and?boiling
- Evaporation?involves the change of liquid to gas, but unlike boiling,?evaporation?occurs only at the surface and takes place at temperatures below the?boiling point
- Boiling?occurs at a specific temperature and takes place when the?vapour pressure?reaches the external atmospheric pressure
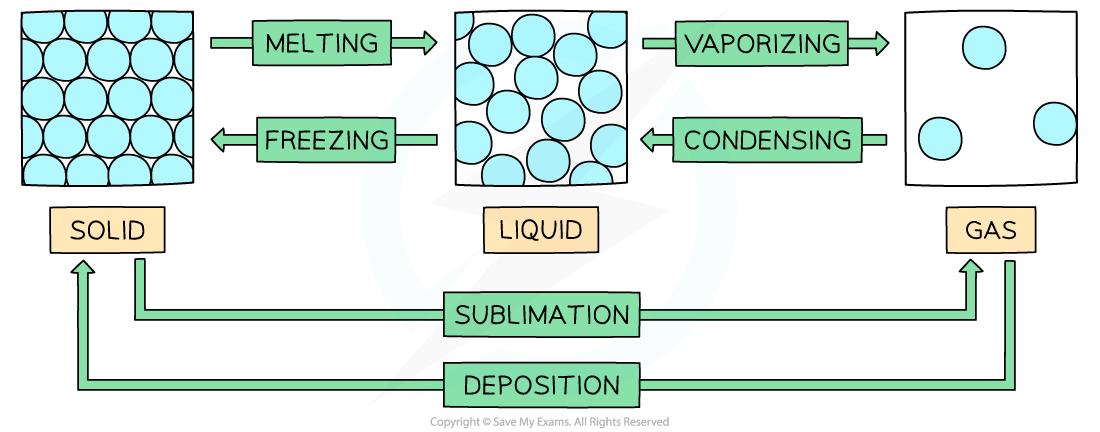
State Changes
- The relationship between temperature and energy during state changes can be represented graphically
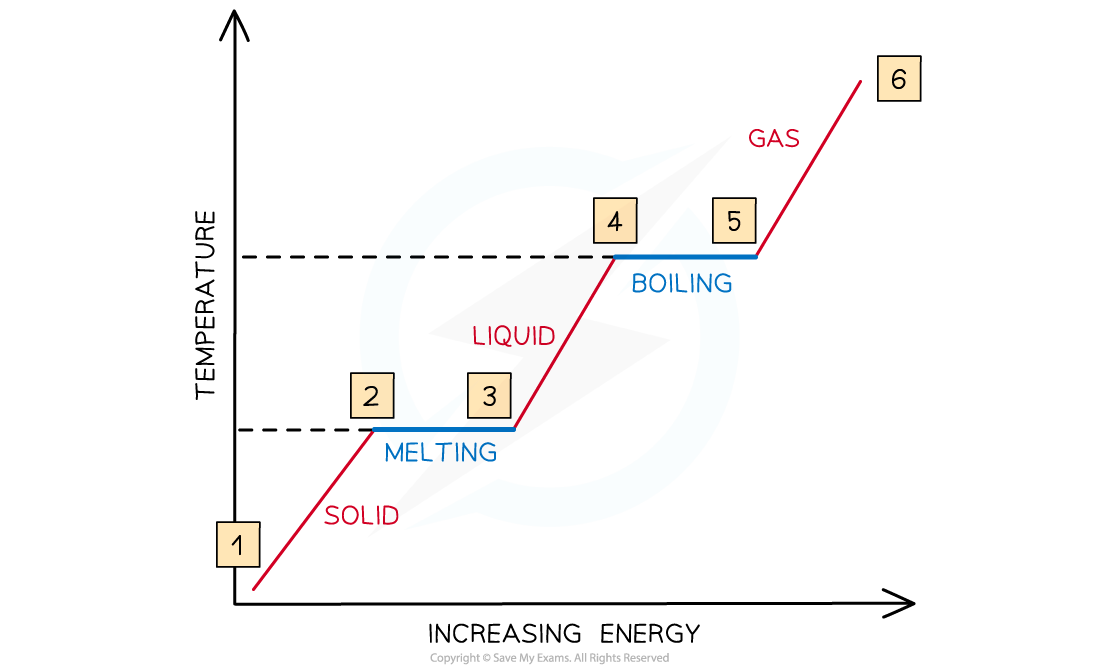
The relationship between temperature and energy during state changes
- Between 1 & 2, the particles are vibrating and gaining?kinetic energy?and?the temperature rises
- Between 2 & 3, all the energy goes into breaking bonds – there is?no?increase in?kinetic energy?or?temperature
- Between 3 & 4, the particles are moving around and gaining in?kinetic energy
- Between 4 & 5, the substance is boiling, so bonds are breaking and there is?no?increase in?kinetic energy?or?temperature
- From 5 & 6, the particles are moving around rapidly and increasing in?kinetic energy
Exam Tip
Be careful to match the bond breaking or making processes to the flow of energy during state changes. Remember that to?break?bonds, energy is always?needed?to overcome the?forces of attraction?between the particles
轉(zhuǎn)載自savemyexams

最新發(fā)布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









