- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
Edexcel IGCSE Chemistry 復習筆記 4.2.1 Crude Oil & Fractional Distillation
Edexcel IGCSE Chemistry 復習筆記 4.2.1 Crude Oil & Fractional Distillation
Crude Oil & Fractional Distillation
- Crude oil as a mixture is not a very useful substance but the different hydrocarbons that make up the mixture, called fractions, are enormously valuable, with each fraction having many different applications
- Each fraction consists of groups of hydrocarbons of?similar?chain lengths
- The fractions in petroleum are separated from each other in a process called?fractional distillation
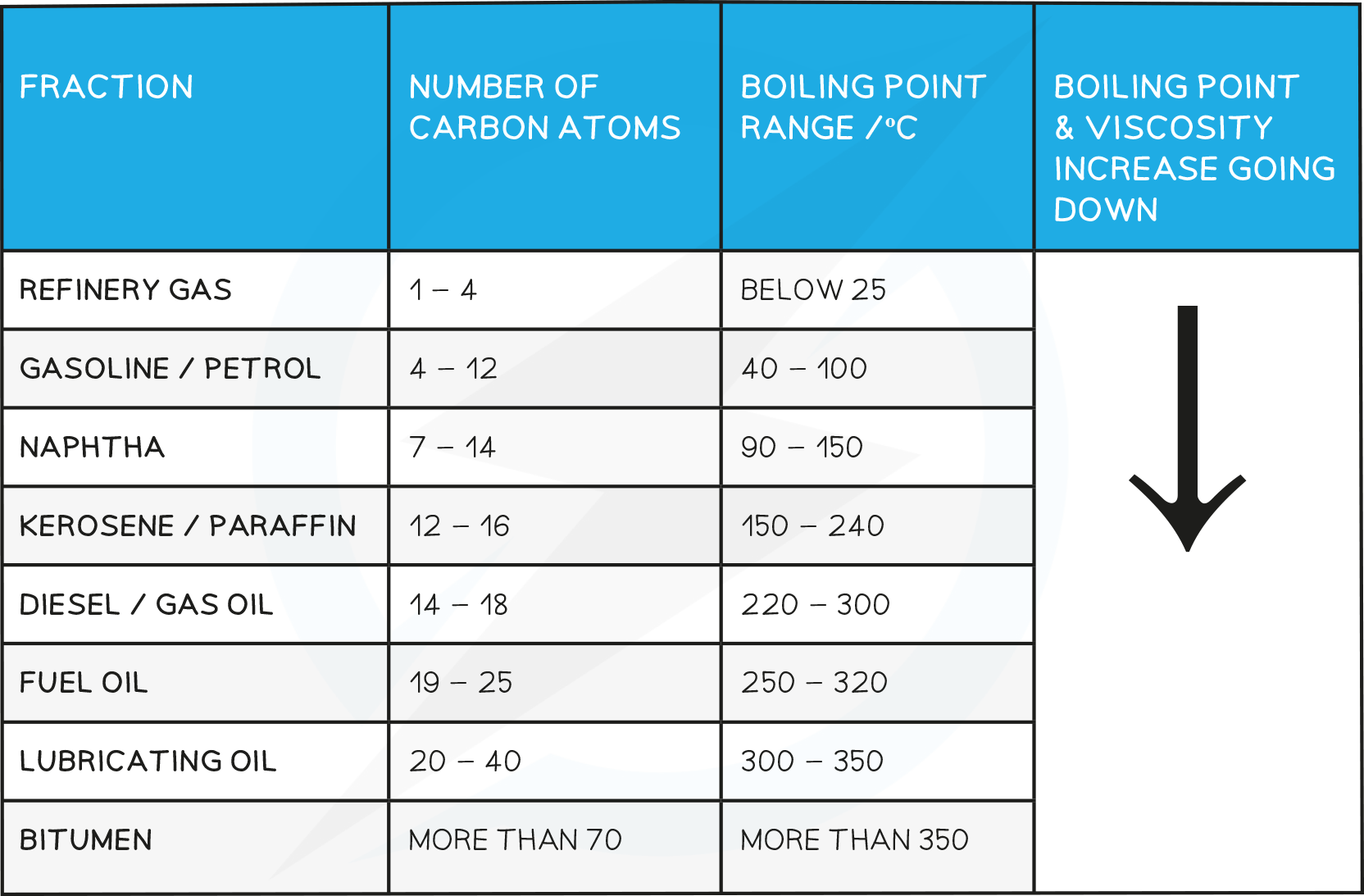
- The molecules in each fraction have similar?properties?and?boiling points, which depend on the number of carbon atoms in the chain
- The size and length of each hydrocarbon molecule determines in which fraction it will be separated into
- The size of each molecule is directly related to how many carbon and hydrogen atoms the molecule contains
- Most fractions contain mainly?alkanes, which are compounds of carbon and hydrogen with only?single?bonds between them
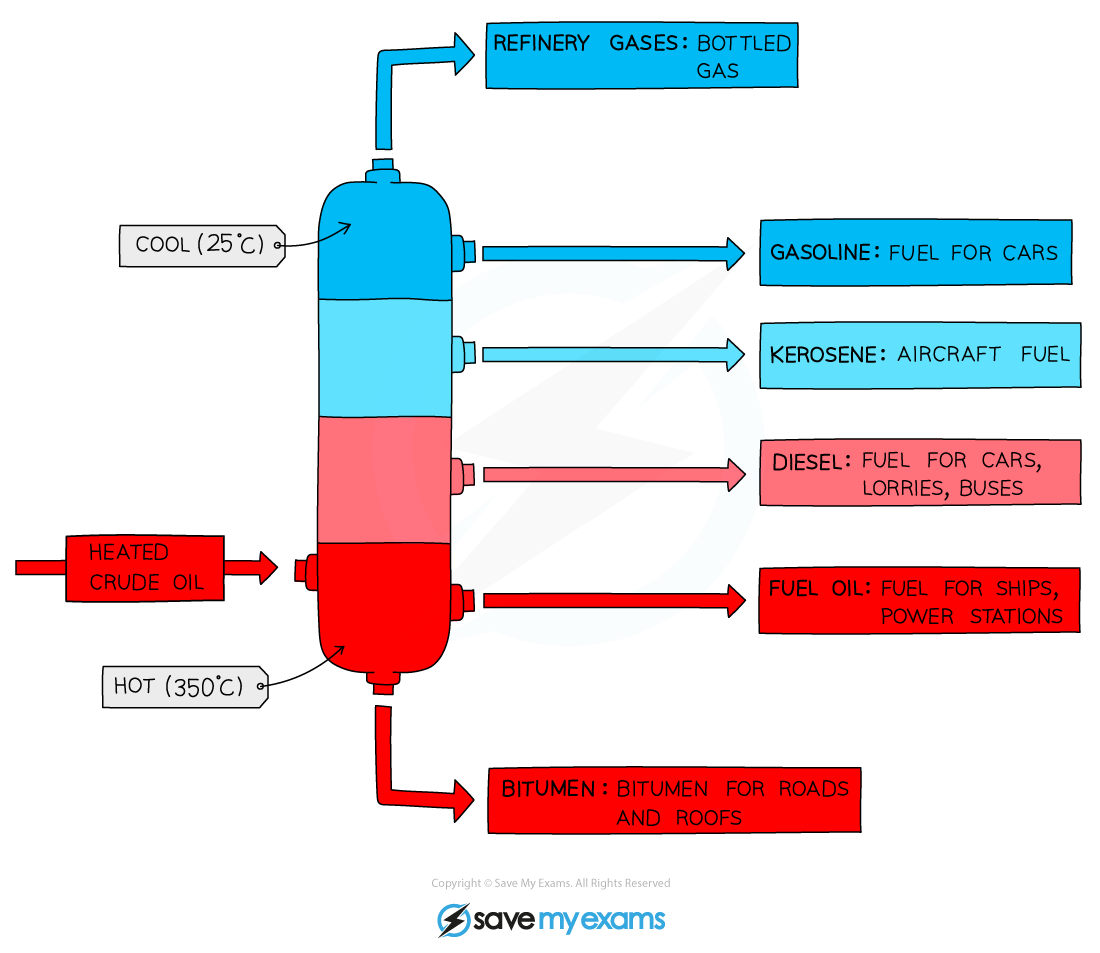
Diagram showing the process of fractional distillation to separate crude oil in a fractionating column
- Fractional distillation is carried out in a?fractionating column?which is very?hot?at the?bottom?and?cool?at the?top
- Crude oil enters the fractionating column and is heated so?vapours rise
- Vapours of hydrocarbons with very?high?boiling points will immediately condense into liquid at the higher temperatures lower down and are tapped off at the bottom of the column
- Vapours of hydrocarbons with?low?boiling points will rise up the column and condense at the top to be tapped off
- The different fractions condense at different heights according to their?boiling points?and are tapped off as liquids
- The fractions containing?smaller?hydrocarbons are collected at the top of the fractionating column as gases
- The fractions containing?bigger?hydrocarbons are collected at the lower sections of the fractionating column
Exam Tip
As you move up a fractionating column the temperature decreases, so the compounds with higher boiling points come off lower down the column.
The Main Fractions
Properties of the main fractions of crude oil
- Viscosity:?This refers to the ease of flow of a liquid. High viscosity liquids are thick and flow less easily. If the number of carbon atoms increases, the attraction between the hydrocarbon molecules also increases which results in the liquid becoming more viscous with the increasing length of the hydrocarbon chain. The liquid flows less easily with increasing molecular mass
- Colour:?As carbon chain length increases the colour of the liquid gets darker as it gets thicker and more viscous
- Melting point/boiling point: As the molecules get larger, the intermolecular attraction becomes greater. So more heat is needed to separate the molecules. With increasing molecular size there is an increase in boiling point
- Volatility:?Volatility refers to the tendency of a substance to vaporise. With increasing molecular size hydrocarbon liquids become less volatile. This is because the attraction between the molecules increases with increasing molecular size
Trend in Boiling Point of the Main Fractions
Uses of the different fractions obtained from petroleum (crude oil)
- The petrochemical industry is hugely important for modern society and development
- The fuels that are used in most modern methods of transport (cars, trains, airplanes etc.) are all based on oil products
- Polymers, lubricants, solvents, detergents and adhesives are all products that are obtained from crude oil
- The array of fractions in crude oil and the huge range of compounds we can produce from them all stem from carbon’s ability to form multiple strong covalent bonds with itself leading a huge number of organic compounds
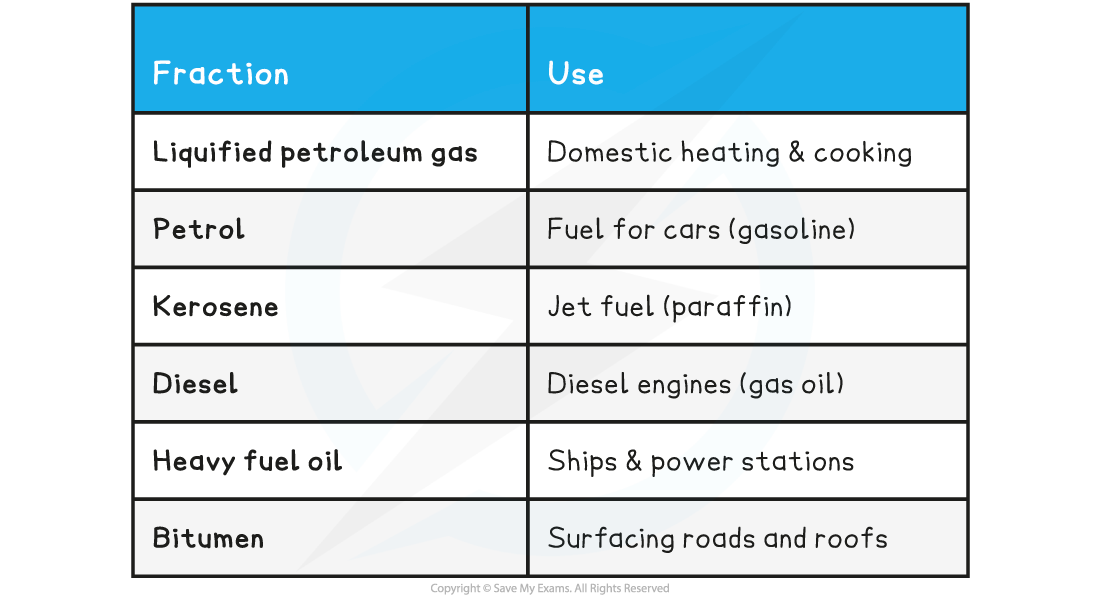
- The main fractions and their uses are described in the following table:
Uses of Crude Oil Fractions
Exam Tip
You need to learn the names and uses of the main fractions obtained from crude oil: refinery gases(also known as liquid petroleum gases), gasoline, kerosene, diesel, fuel oil and bitumen. Gasoline and petrol are the same thing; gasoline is the term used in the USA.
轉載自savemyexams

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









