- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
Edexcel IGCSE Chemistry 復習筆記 2.8.3 Tests for Cations
Edexcel IGCSE Chemistry 復習筆記 2.8.3 Tests for Cations
Tests for Cations
- Metal cations in aqueous solution can be identified by the?colour?of the precipitate they form on addition of sodium hydroxide and ammonia
- If only a small amount of NaOH is used then normally the?metal hydroxide?precipitates
?Analysing results
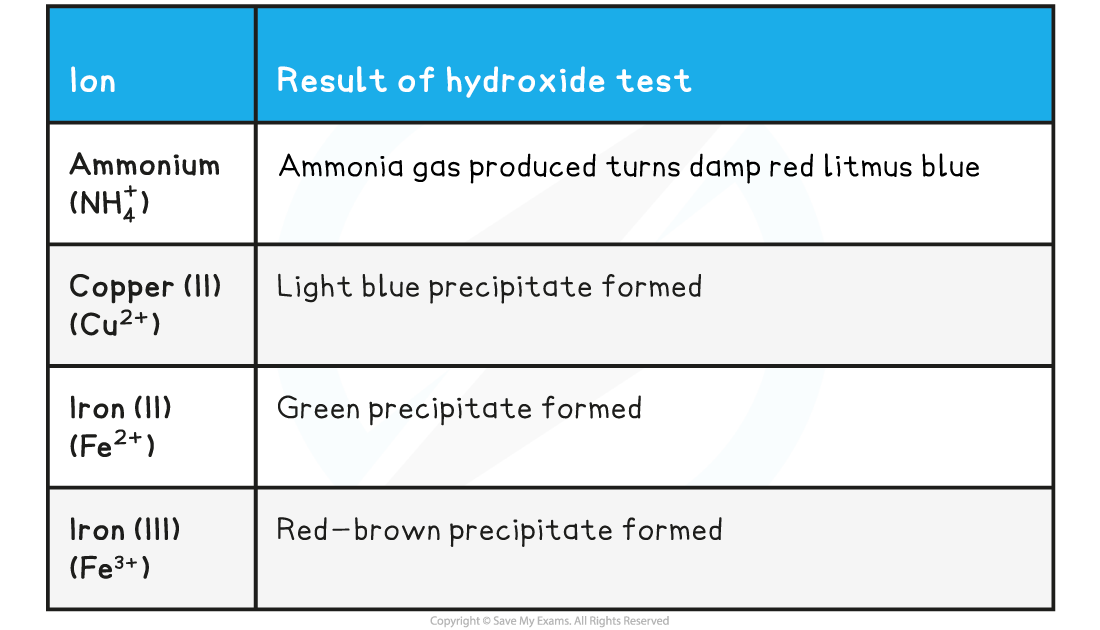
- The table below contains the results for each of the cations included in the syllabus
- If a precipitate is formed from NaOH then the hydroxide is insoluble in water
Testing for Cations Table
Exam Tip
Sometimes you may not see much of a precipitate because the cation you are testing is present is very small amounts. However, every a slight cloudiness or colour change can indicate a positive test result.
轉載自savemyexams

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









