- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
AQA A Level Chemistry復習筆記7.3.4 Ester Hydrolysis
Ester Hydrolysis
Hydrolysis of Esters - Acid
- The reverse of the esterification reaction is called?hydrolysis
- Ester hydrolysis is a useful reaction for creating biodegradable plastics
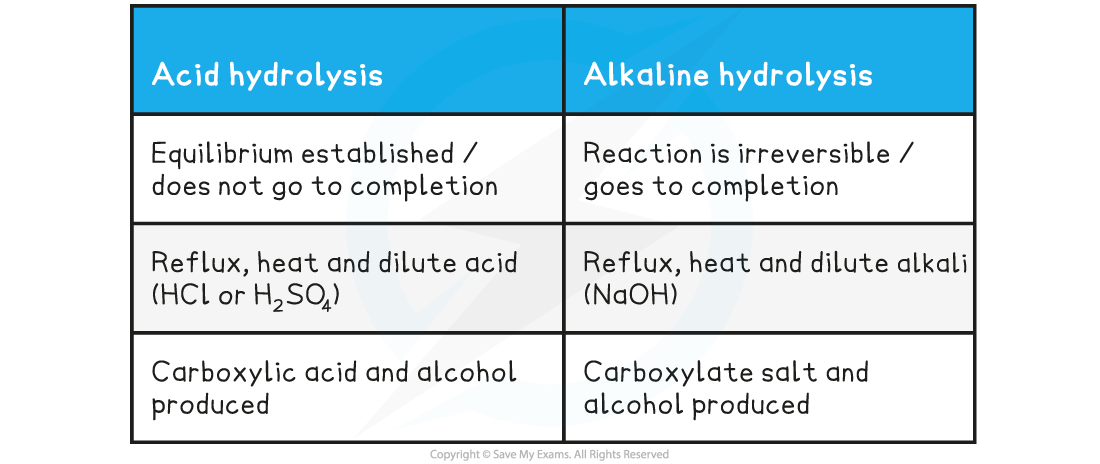
- Esters can be?hydrolysed?to reform the carboxylic acid and alcohol or salts of carboxylic acids by using either?dilute?acid?(e.g. sulfuric acid) or?alkali?(e.g. sodium hydroxide) and?heat
- When an ester is?heated under reflux?with?acid?an equilibrium mixture is established, meaning that the hydrolysis reaction is not complete
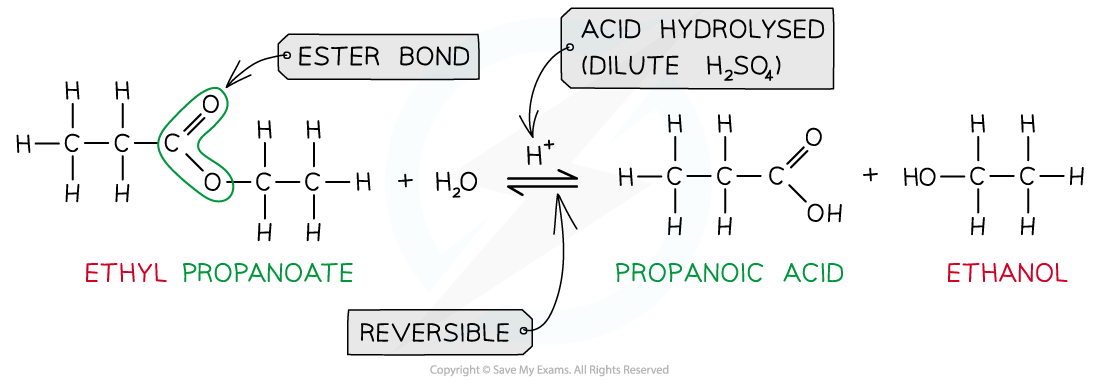
Ester hydrolysis by dilute acid is a reversible reaction forming carboxylic acid and alcohol
Hydrolysis of Esters - Alkaline
- However,?heating?the ester?under reflux?with?dilute alkali?(e.g. sodium hydroxide) is an?irreversible?reaction as the ester is fully hydrolysed and the reaction goes to completion
- The carboxylic acid produced reacts with excess alkali to form a?carboxylate salt?and?alcohol
- The?sodium?carboxylate?salt requires further?acidification?to turn into a?carboxylic?acid
- The sodium carboxylate (-COO-) ion needs to get protonated by an acid (such as HCl) to form the carboxylic acid (-COOH)
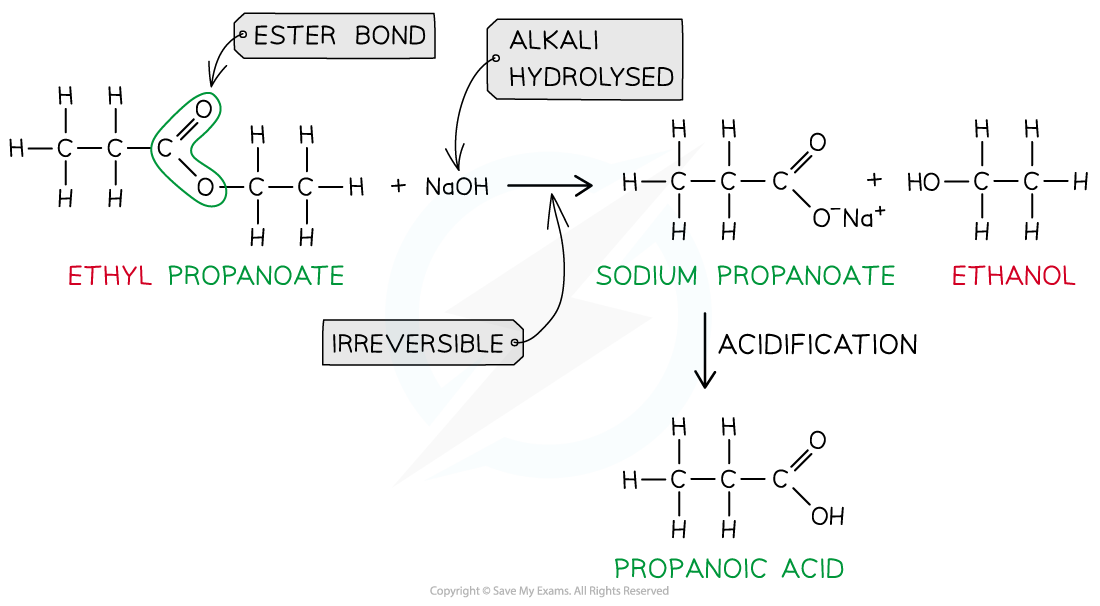
Ester hydrolysis by dilute alkali is an irreversible reaction forming a sodium carboxylate salt and alcohol
Table showing differences in hydrolysis of esters
Worked Example
Name the products and write equations for the following hydrolysis reaction:
- Ethyl ethanoate with hot dilute sulfuric acid solution
- Methyl propanoate by hot sodium hydroxide solution
Answer:
Answer 1:?Ethanoic acid and ethanol
CH3COOCH2CH3?+ H2O ? CH3COOH + CH3CH2OH
Answer 2:?Sodium propanoate and methanol
CH3CH2COOCH3?+ NaOH → CH3CH2COONa + CH3OH
Making Soap
Soaps
- Vegetable oils and animal fats can be hydrolysed in?alkaline?conditions with?aqueous sodium hydroxide?to form soaps
- The process is also called?saponification
- Soaps are?carboxylate salts?of long-chain carboxylic acids, known as fatty acids
- When triglycerides / fats are hydrolysed in hot alkaline conditions, the product is a mixture containing glycerol (propane-1,2,3-triol) and the salts of the fatty acids, soaps
轉載自savemyexams

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









