- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
AQA A Level Chemistry復習筆記5.1.3 Born-Haber Calculations
Calculations Using Born-Haber Cycles
- Once a Born-Haber cycle has been constructed, it is possible to calculate the lattice energy (ΔHlatt?) by applying Hess’s law and rearranging:
ΔHf??= ΔHat??+ ΔHat??+?IE?+?EA?+ ΔHlatt?
- If we simplify this into three terms, this makes the equation easier to see:
- ΔHlatt?
- ΔHf?
- ΔH1??(the sum of all of the various enthalpy changes necessary to convert the elements in their standard states to gaseous ions)
- The simplified equation becomes
ΔHf??= ΔH1??+ ΔHlatt?
So, if we rearrange to calculate the lattice energy, the equation becomes
ΔHlatt??= ΔHf??- ΔH1?
- When calculating the ΔHlatt?, all other necessary values will be given in the question
- A Born-Haber cycle could be used to calculate any stage in the cycle
- For example, you could be given the lattice energy and asked to calculate the enthalpy change of formation of the ionic compound
- The principle would be exactly the same
- Work out the?direct?and?indirect route?of the cycle (the stage that you are being asked to calculate will always be the direct route)
- Write out the equation in terms of enthalpy changes and rearrange if necessary to calculate the required value
- Remember:?sometimes a value may need to be doubled or halved, depending on the ionic solid involved
- For example, with MgCl2?the value for the first electron affinity of chlorine would need to be doubled in the calculation, because there are two moles of chlorine atoms
- Therefore, you are adding?2 moles?of electrons to?2 moles?of chlorine atoms, to form?2 moles?of Cl-?ions
Worked Example
Calculating the lattice energy of KClGiven the data below, calculate the ΔHlatt??of potassium chloride (KCl)??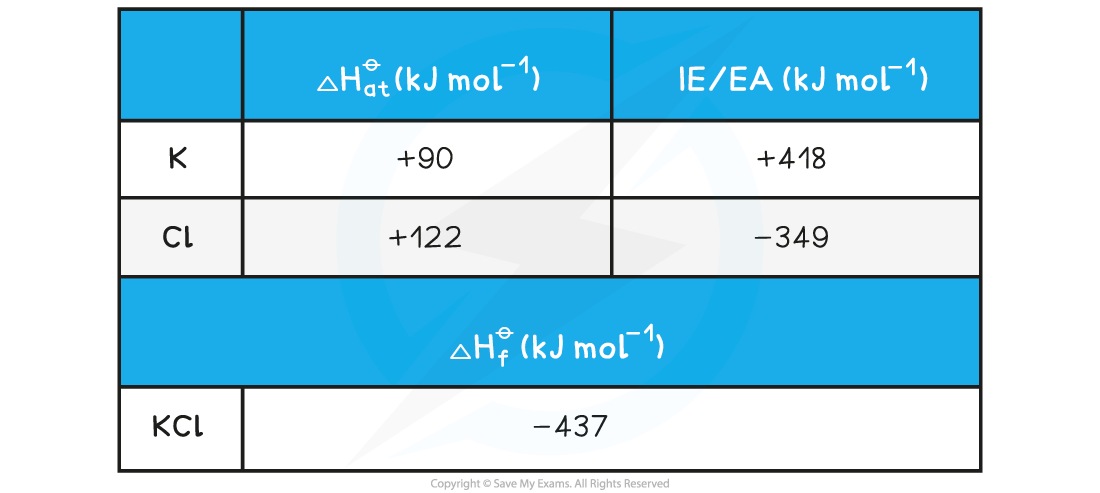
Answer
Step 1:?The corresponding Born-Haber cycle is:
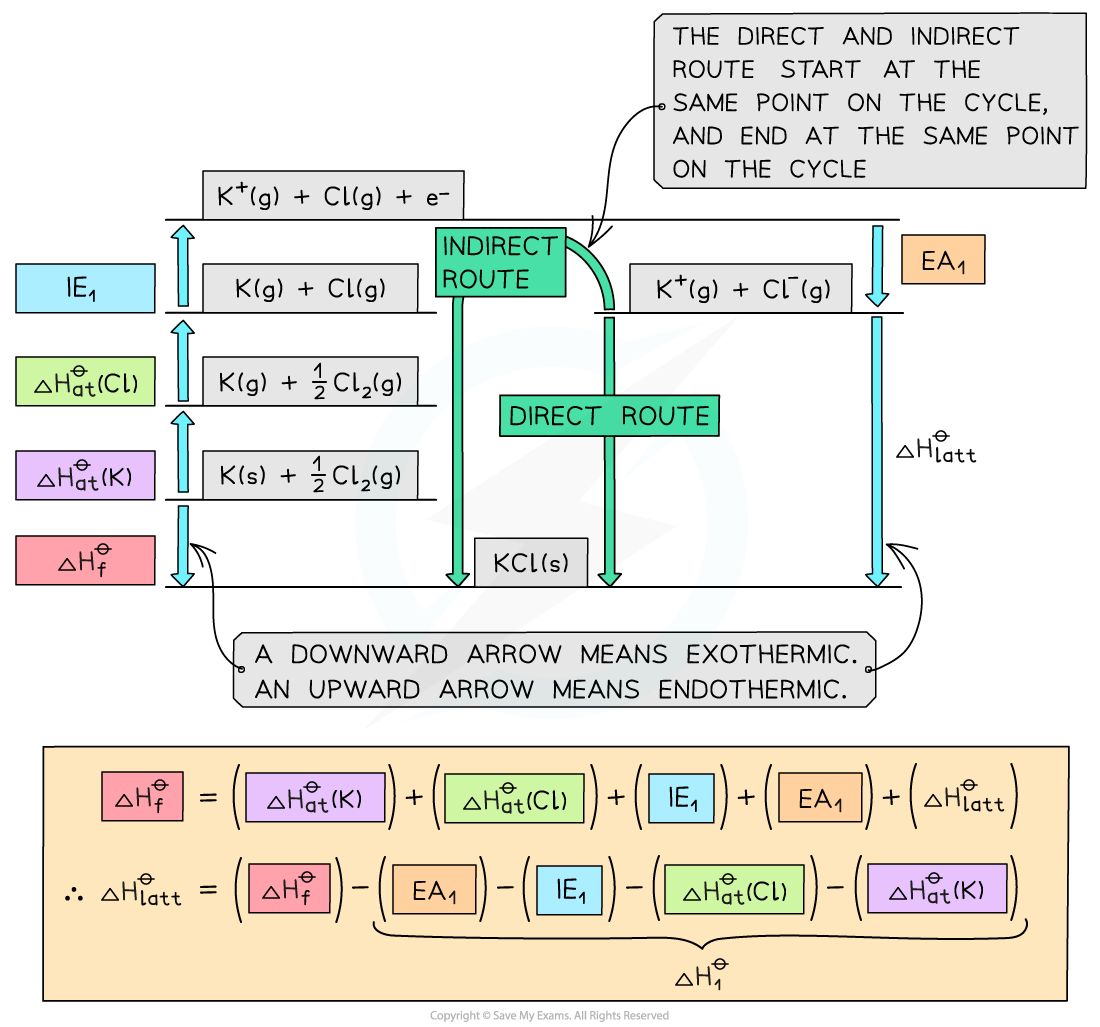
Step 2:?Applying Hess’ law, the lattice energy of KCl?is:
ΔHlatt??= ΔHf??- ΔH1?
ΔHlatt??= ΔHf??- [(ΔHat??K) + (ΔHat??Cl) + (IE1?K) + (EA1?Cl)]
Step 3:?Substitute in the numbers:
ΔHlatt??= (-437) - [(+90) + (+122) + (+418) + (-349)]?= -718 kJ mol-1
Worked Example
Calculating the lattice energy of MgOGiven the data below, calculate the of ΔHlatt??magnesium oxide of magnesium oxide (MgO)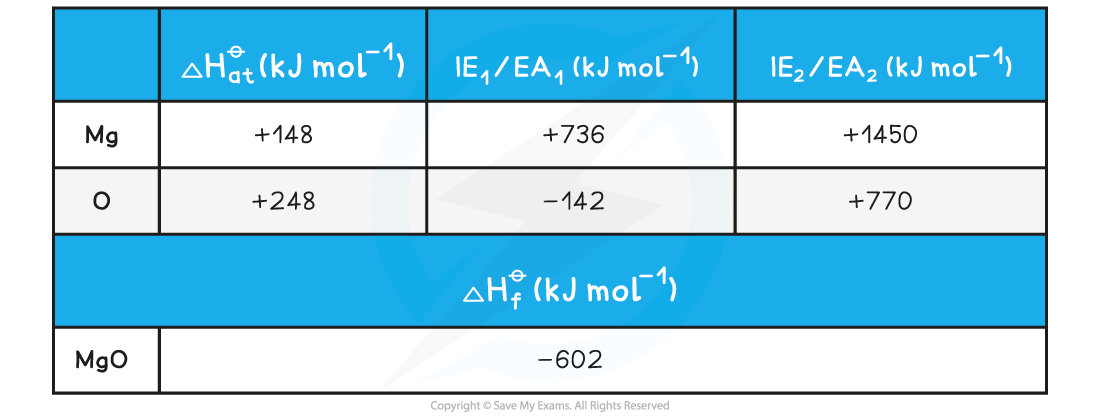
Answer
Step 1:?The corresponding Born-Haber cycle is:
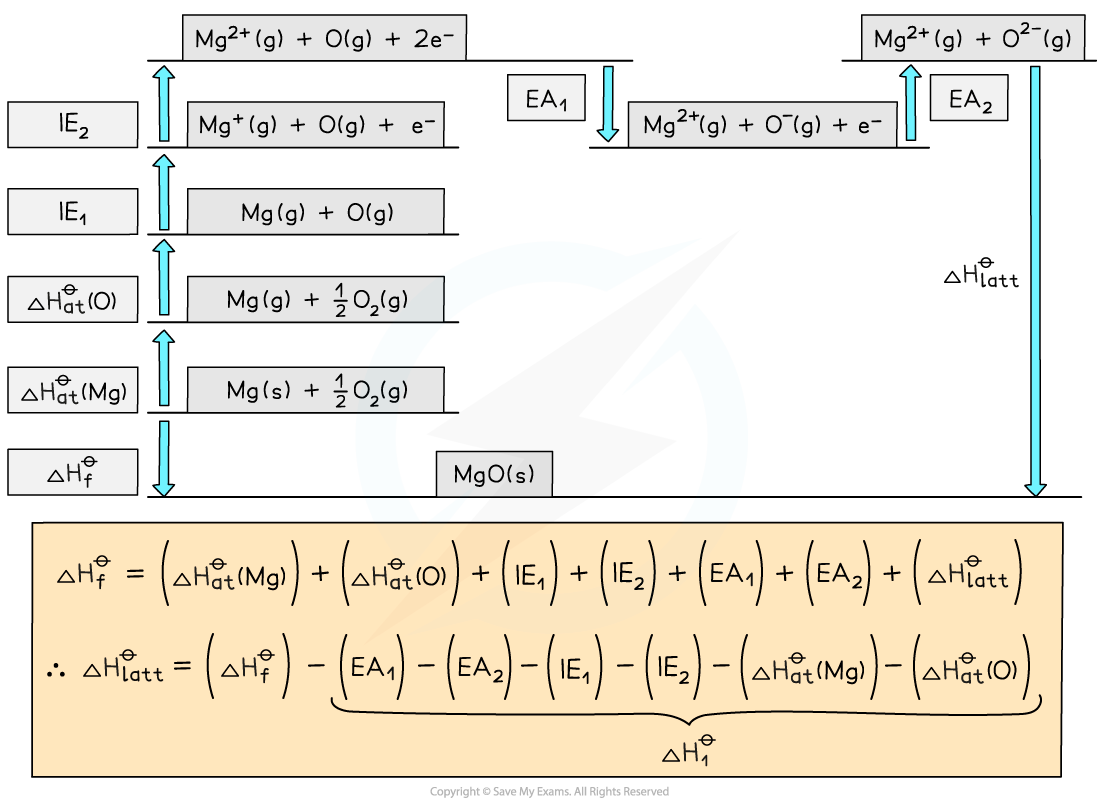
Step 2:?Applying Hess’ law, the lattice energy of MgO is:
ΔHlatt??= ΔHf??- ΔH1?
ΔHlatt??= ΔHf??- [(ΔHat??Mg) + (ΔHat??O) + (IE1?Mg) + (IE2?Mg) + (EA1?O) + (EA2?O)]
Step 3:?Substitute in the numbers:
ΔHlatt??= (-602) - [(+148) + (+248) + (+736) + (+1450) + (-142) + (+770)]
= -3812 kJ mol-1
Exam Tip
Make sure you use brackets when carrying out calculations using Born-Haber cycles as you may forget a +/- sign which will affect your final answer!
轉載自savemyexams

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









