- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
AQA A Level Chemistry復習筆記3.7.1 Fundamentals of Reaction Mechanisms
Reaction Mechanisms: Terminology
Homolytic & Heterolytic fission
- Homolytic fission?is breaking a covalent bond in such a way that each atom takes an electron from the bond to form two radicals
- Heterolytic fission?is breaking a covalent bond in such a way that the more electronegative atom takes both the electrons from the bond to form a negative ion and leaving behind a positive ion
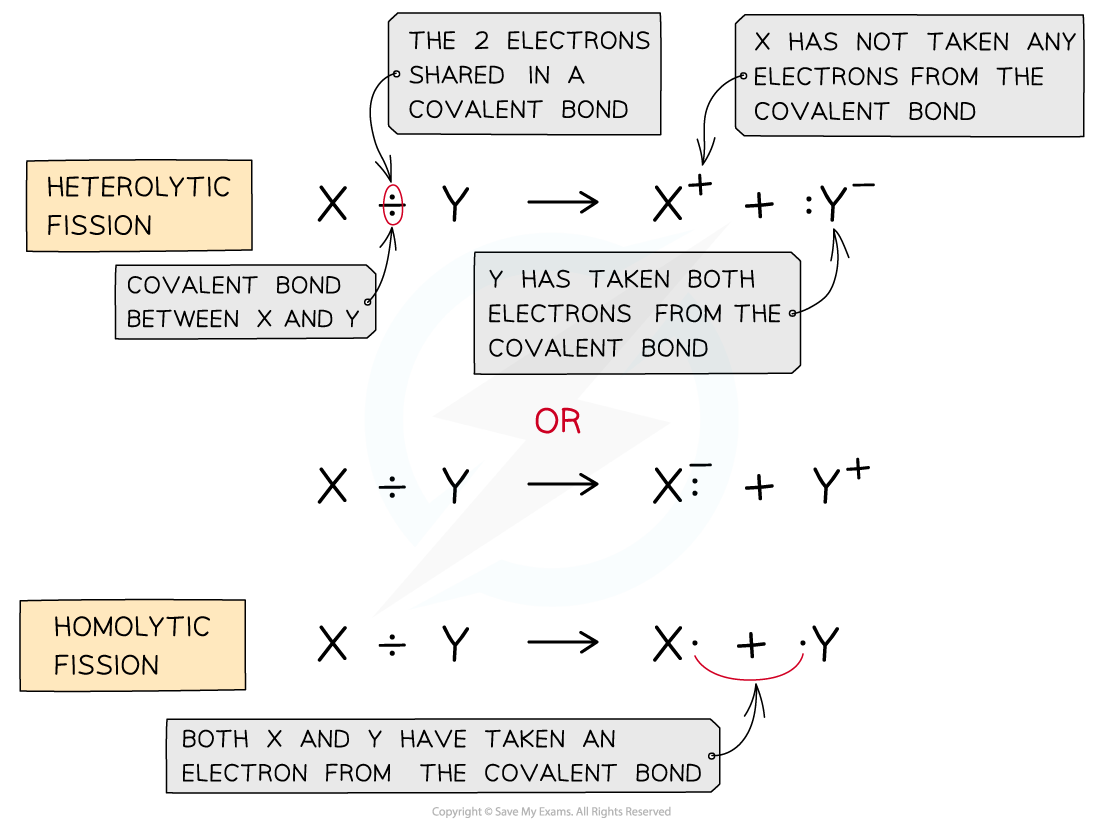
The diagram shows heterolytic fission in which the most electronegative atom takes both electrons in the covalent bond and homolytic fission in which each atom takes one electron from the covalent bond
Nucleophiles & electrophiles
- A?nucleophile?is an electron-rich species that can?donate?a pair of electrons
- ‘Nucleophile’ means ‘nucleus/positive charge loving’ as nucleophiles are attracted to positively charged species
- Nucleophilic?refers to reactions that involve a nucleophile
- An?electrophile?is an electron-deficient species that can?accept?a pair of electrons
- ‘Electrophile’ means ‘electron/negative charge loving’ as electrophiles are attracted to negatively charged species
- Electrophilic?refers to reactions that involve an electrophile

A nucleophile ‘loves’ a positive charge and an electrophile ‘loves’ a negative charge
Types of reactions
- An?addition?reaction is an organic reaction in which two (or more) molecules combine to give a?single?product?with no other products
- A?substitution?reaction is a reaction that involves?replacing?an atom or group of atoms by another
- An?elimination?reaction is a reaction in which a small molecule (such as H2O or HCl) is?removed?from an organic molecule
- A?hydrolysis?reaction is a reaction in which a compound is?broken?down?by?water?(it can also refer to the breakdown of a substance by dilute acids or alkali)
- A?condensation?reaction is a reaction in which two organic molecules join together and in the process?eliminate?small molecules (such as H2O or HCl)
Oxidation & reduction
- An?oxidation?reaction is a reaction in which oxygen is added, electrons are removed or the oxidation number of a substance is increased
- In organic chemistry it often refers to the addition of oxygen or removal of hydrogen atoms to a substance
- In equations for organic redox reactions, the symbol [O] can be used to represent one atom of oxygen from an oxidising agent
- A?reduction?reaction is a reaction in which oxygen is removed, electrons are added or the oxidation number of a substance is decreased
- In organic chemistry it often refers to the removal of oxygen or addition of hydrogen atoms to a substance
- In equations for organic redox reactions, the symbol [H] can be used to represent one atom of hydrogen from a reducing agent
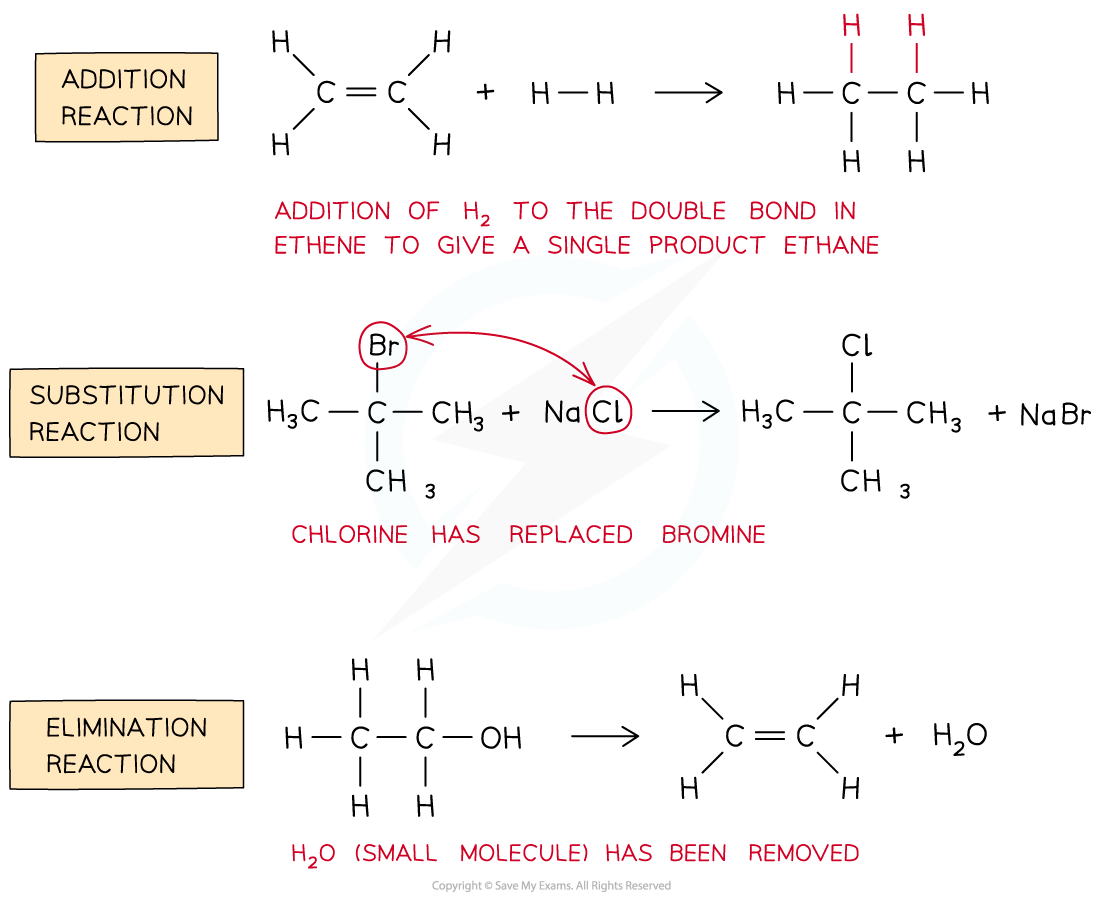
Different types of reactions in organic chemistry
Fundamentals of Mechanisms
- In organic reaction mechanisms,?curly?arrows?represent the movement of?electron?pairs
- The arrow beings at a bond or a lone pair of electrons and points to the species that accepts the lone pair of electrons
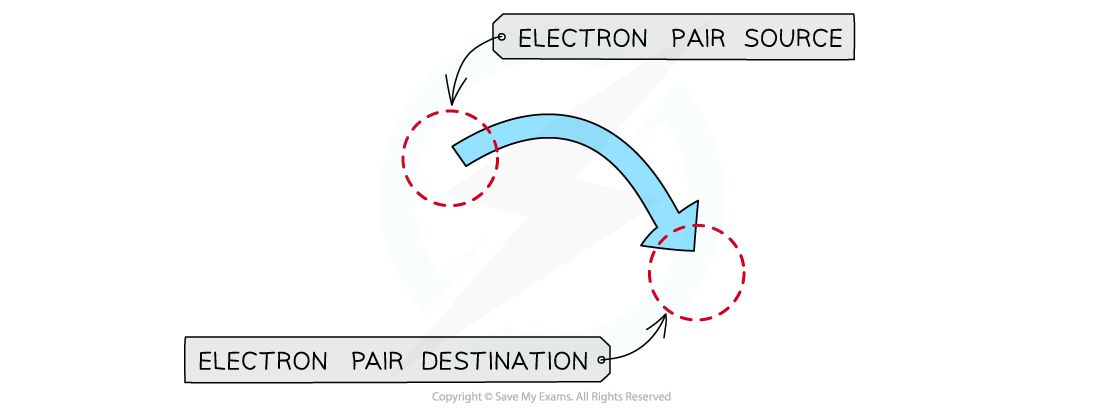
Curly arrows show electron pairs moving from the source (e.g. a nucleophile) to its destination (e.g. an electrophile)
轉載自savemyexams

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









